Lab - Week One: The Scientific Method
advertisement

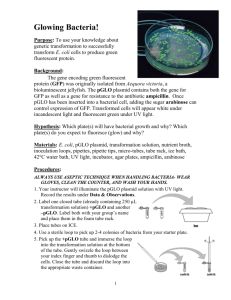
Biology 211 - Lab Nine: Gene Regulation Reminder: This lab requires a 1-hour follow-up 15-24 hours after your lab. Pre-Lab Homework: 1. Define transformation, including who is being transformed and for what trait. 2. Name the plasmid we are using in this lab; identify the purpose of the following key DNA regions on this plasmid: ori, GFP, amp, and ara. 3. Based on lecture, describe what positive vs. negative gene regulation is? What kind of regulation is going on in this exercise? Explain. 4. What does the acronym BLAST stand for and what does BLAST do? In-Lab Activity Overview a. Transformation b. Gene Regulation c. Analysis of Gene Sequences Using BLAST d. 15-24 Hour Follow-Up e. NCBI/BLAST Extra Credit ara promoter amp Transformation – work in pairs. This exercise is from the Biotechnology Explorer – pGLO Transformation™ kit published by BIO-RAD. In this experiment you will be transforming E. coli using cold CaCl2 and a process called heat-shock. Specifically, you will transform bacteria with the pGLO plasmid. Like all plasmids, pGLO contains its own origin of replication (ori). Like many plasmids, pGLO also contains a gene for antibiotic resistance (amp) – specifically, to the drug ampicillin. Recombinant DNA technologists have also constructed pGLO to contain a gene that is made up of GFP driven by a promoter regulated by araC. GFP stands for “green fluorescent protein” and comes from a mystery source you will explore later. We will talk about araC in the next section. After the procedure, if conditions are correct and the bacteria express their newly acquired genes, they will glow a brilliant green color under ultraviolet light. SAFETY: PUT ALL ITEMS THAT HAVE TOUCHED (OR MAY HAVE TOUCHED) BACTERIA IN THE BIOHAZARD CONTAINER AT YOUR TABLE!!! Mixing Procedures – Work In Pairs 1. Label closed tubes +pGLO and –pGLO, and your pair’s name or initials. 2. Using a sterile transfer pipette, add 0.25 ml CaCl2 into each tube. Place on ice! 3. Using a sterile loop, pick up THREE bacterial colonies from starter plate. Pick Biology 211 Lab Manual, Lab Nine, page 1 up +pGLO tube and immerse loop in CaCl2 solution. Spin loop between your index finger and thumb until cells are dispersed (no chunks!). Place tube on ice. 4. Using new sterile loop, repeat for –pGLO tube, close the lid, and place on ice. 5. Immerse a new sterile loop into the pGLO plasmid stock tube. When you withdraw the loop, you should see a film of DNA solution across the ring. Mix the loop of DNA into the bacterial suspension in the +pGLO tube by spinning as above. Close the tube and return it to the ice. DO NOT add plasmid to the – pGLO tube – Why? 6. Leave the tubes sitting in ice for 10 minutes. Make sure to push the tubes all the way down so that the bottoms of the tubes have good contact with the ice. 7. While the tubes are sitting on ice, label your four different LB nutrient agar plates on the food-containing bottom (not the empty lid) as follows: What You Need One LB/amp plate Second LB/amp plate One LB/amp/ara plate One LB plate Striping to Look For 2 stripes 2 stripes 3 stripes 1 stripe How to Label +pGLO, LB/amp -pGLO, LB/amp +pGLO, LB/amp, ara -pGLO, LB Heat-Shock Transformation and Plating Procedures – Work In Pairs 1. Transfer the +pGLO and -pGLO tubes to the 42°C water bath for EXACTLY 50 seconds. Make sure the tubes stay immersed in the water for the entire time. Following heat-shocking, immediately place the tubes back on ice for 2 minutes. 2. Remove tubes from the ice and place in the rack on your bench. Open +pGLO tube and, using a new sterile pipette, add 0.25 ml LB nutrient broth – close lid. Using a new sterile pipette, repeat for the –pGLO tube. Invert several times to mix. Leave the tubes in the rack at room temperature for 10 minutes. 3. Using a new/different sterile swab for each plate, dip each swab into appropriate tubes (see diagram below) to saturate with bacteria and then spread thoroughly over the surface of the appropriate plate. DO NOT PRESS TOO HARD! Biology 211 Lab Manual, Lab Nine, page 2 4. Stack plates and tape together, labeling with your names and lab time. Place the stack of plates food-side UP in the 37˚C incubator until the next day. Remember that this lab requires a 1-hour follow-up 15-24 hours after your lab. Gene Regulation In bacteria like E. coli, groups of related genes are clustered together in OPERONS and transcribed into RNA from a single promoter. One example is the arabinose (ara) operon, which has been recombined with GFP in today’s pGLO plasmid. Before we consider ara’s role in pGLO, however, let’s talk about what the ara operon does naturally for bacteria. The purpose of the ara operon is to make three enzymes (araB, araA and araD) that digest the sugar arabinose for energy. As should be evident below, the ara operon looks like the lac operon. In a similar manner to CAP-cAMP activating the lac operon, the ara operon can also be regulated through POSITIVE regulation. Specifically, the DNA-binding protein called araC, when allosterically bound by arabinose, POSITIVELY attracts RNA Pol to just upstream of the ara operon promoter. On the recombinant pGLO plasmid, you should observe that there is both an araC gene and an araC-driven promoter upstream of the GFP gene. Complete worksheet question 1a to better understand this system. 15-24 Hour Follow-Up Bacteria like E. coli take between 15-20 hours to multiply to the point that you can physically see their colonies on a plate. Therefore, you need to schedule a time to come back to lab that is between 15-20 hours AFTER your lab. Given issues last year, we are requiring that each INDIVIDUAL come to lab and turn in his/her own worksheet this year. If you want to coordinate and come with your pair partner, that is fine – but worksheets must be completed individually. Procedures For Checking Your Plates Biology 211 Lab Manual, Lab Nine, page 3 1. Find your stack of four plates in the incubator and pull them out. 2. Separate the plates in your stack and examine each one. Observe and record if there are bacteria growing on each plate and whether you can see individual colonies (creamy-white dots) or a complete lawn (widespread creamy-white film). 3. For all plates that have bacterial cells growing on them, place each plate on the UV box (located on the counter next to the incubator), close the lid, and turn on the UV light. Turning off the room lights will help with visualization. Glowing cells will fluoresce green (others will look yellowish). 4. When you are finished, re-tape your plate stack and return them to the incubator. ALL plates will be discarded after 5 p.m. on the day after your lab – 5 p.m. is also when your reports are due to your lab instructor! Analysis of Gene Sequences Using BLAST As the sequencing of genomes becomes more common and more efficient, several online databases have been developed that allow you to search for genes by their DNA or protein sequence. One such example is a program called BLAST (Basic Local Alignment Search Tool) maintained by the database GenBank, used to input a known sequence and find all similar sequences. Here, you will use an actual DNA sequence to search BLAST and determine the most similar genes. Procedures – Gene Sequence Analysis Application 1. Go to a lab computer and enter the site http://blast.ncbi.nlm.nih.gov/Blast.cgi Under the Basic BLAST list, click on “nucleotide blast.” 2. Logon to your course website and open the document with your mystery BLAST sequence – copy the entire DNA sequence. 3. Paste the copied mystery sequence into the BLAST window that says “Enter accession number, gi, or FASTA sequence” 4. Where it says “Choose Search Set”, click the choice for “others” and make sure the box below states “Nucleotide collection (nr/nt)” 5. Under “Program Selection,” choose “Highly similar sequences (megablast)” 6. Finally, click the button that says “BLAST” and wait a few minutes for results. 7. The first part of the results page is a visual representation of sequences similar to the one that you input. Scroll down the page and you will find a list of clickable sequence hits. Click on the links under “Accession” to get more information about each gene hit. Biology 211 Lab Manual, Lab Nine, page 4 NCBI/BLAST – 5 pts. Extra Credit You are an evolutionary biologist who wants to use DNA sequence information to examine the relationship between diverse living things. Using ideas from this course, find a gene that ALL living things must have. 1. Use NCBI to find a sequence of this gene from the following organisms – which represent ALL three DOMAINS of life: Escherichia coli (E. coli) – BACTERIA Drosophila (fruit fly, any species) – EUKARYA Sulfolobus (any species) – ARCHAEA 2. Print out each gene sequence above – JUST your target gene (should be 1-3 pages at the most!); be careful when printing because you might find that your gene sequence is embedded in a MASSIVE genome file and that would be dozens of pages long. 3. Choose any one sequence above and use it to do a BLAST that is targeted against the Human Genome. Print out the first THREE PAGES (ONLY) of your hitlist. Biology 211 Lab Manual, Lab Nine, page 5 Lab Nine: Gene Regulation - Turn In INDIVIDUALLY, 5 p.m. Day After Your Lab Name: 1. Work Through the Following to Better Understanding pGLO/ara Gene Regulation: a. This table is worth 3.5 pts. For every mistake, 0.5 pts. will be subtracted! CONDITION araC Allosteric Site Answer The Following! araC DNA Site Promoter Arabinose PRESENT a. empty b. bound by _________? a. empty b. bound by _________? a. empty b. bound by _________? Arabinose ABSENT a. empty b. bound by _________? a. empty b. bound by _________? a. empty b. bound by _________? b. 4 pts. Explain the purpose of your plates AND predict whether each will glow. Explain Purpose Glow? +pGLO LB/amp -pGLO LB/amp +pGLO LB/amp, ara -pGLO LB c. 0.5 pts. Very often an organism’s traits are the product of both its genes and its environment (“nature” and “nurture”). What two factors must be present in the bacteria’s environment for you to see the glowing green color? Biology 211 Lab Manual, Lab Nine, page 6 2. 1.5 pts. Complete the following about your BLAST exercise: a. What is the name of the gene? b. What organism is your gene sequence most similar to? c. What other sources are also similar to your gene sequence? 3. 2 pts. Follow-Up Observations About Transformation Plates: Describe Bacterial Growth Glow? +pGLO LB/amp -pGLO LB/amp +pGLO LB/amp, ara -pGLO LB 4. If you chose to do the extra credit, make sure it is all stapled to this report! Biology 211 Lab Manual, Lab Nine, page 7