Introductory Microbiology Chap. 5 Outlines Microbial Metabolism I
advertisement

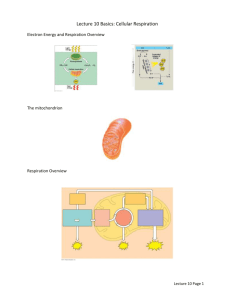
Introductory Microbiology Chap. 5 Outlines Microbial Metabolism I. II. III. IV. V. VI. VII. VIII. IX. X. XI. Catabolic and Anabolic Reactions Enzymes Energy Production Carbohydrate Metabolism Lipid and Protein Catabolism Biochemical Tests and Bacterial Identification (Lab, not on lecture exam) Photosynthesis A Summary of Energy Production Mechanisms Metabolic Diversity among Organisms Metabolic Pathways of Energy Use The Integration of Metabolism Introduction What basic things do living organisms have to acquire in order to live? What determines what molecules or energy sources a specific species needs or is able to use? All living cells’ energy ‘currency’: Term: Phosphorylation Note: Cells use only two kinds of energy: 1) light energy: trapped and used by plants, algae, and some bacteria for photosynthesis and 2) chemical energy: the energy held in the bonds of various chemicals. Cells do not use thermal or electrical energy because they don't have thermal or electrical converters. Thermal potential (that is, temperature) affects the rate of chemical reactions, but does not provide any energy. What about the electrical signals of nervous impulses? The cells use energy in the form of ATP to generate electric potentials in the membrane of nerve cells and fibers. Those electrical signals are not ‘used’ by the cell to perform other work. 1 Most metabolic processes are ‘similar’ in all cells, but not all. Microbes can do things we cannot do! Crazy things, like eat petroleum or radioactive materials and things that are waste products to us. On the other hand, there are microbes that cannot live in our environment, such as those that are killed by oxygen. I. Catabolic and Anabolic Reactions LO/CYU 5-1 A. METABOLISM : Two categories of Chemical Processes: Catabolic and Anabolic Reactions Metabolic ‘Pathways’ 2 B. Molecules and Energy- intertwined…. LO/CYU 5-2 Role of ATP Fig. 2.18 on p. 49 3 II. Enzymes: How they function and what can affect them Enzymes increase the rate of chemical reactions by decreasing the activation energy required for that specific reaction. LO/CYU 5-4 A. Collision Theory The collision theory states that chemical reactions occur when atoms, ions, and molecules collide Activation energy is the amount of energy needed for them to collide ‘hard’ enough to disrupt electronic configurations and produce a chemical reaction Reaction rate is the frequency of collisions with enough energy to bring about a reaction. Reaction rate can be increased by enzymes or by increasing temperature or pressure B. Enzymes and Chemical Reactions Catalysts Substrate(s) Product(s) Decrease activation energy C. Enzyme Specificity and Efficiency Specificity The turnover number is generally 1 to 10,000 molecules per second D. Naming Enzymes See Table 5.1 p. 116 Enzyme Classification Based on Type of Chemical Reaction Catalyzed 4 E. Enzyme Components Fig. 5.3 p. 116 LO/CYU 5-3 Apoenzyme (protein portion) Cofactor (nonprotein portion) Holoenzyme LO/CYU 5-4 F. The Mechanism of Enzymatic Action: The sequence of events in enzyme action on the reactant(s), the enzyme’s substrate(s). Lock and Key Model Fig. 5.4 p. 118 5 G. Factors Influencing Enzyme Activity Fig. 5.7 p. 120 End of Chap. ‘Study Questions’ Review #2 a-c & Critical Thinking #2 LO/CYU 5-5 See ARA Assignment #2 III. Energy Production LO/CYU 5-8 A. Introduction 1. Energy is found in the in nutrient molecules. that form bonds between the atoms 2. Why is ATP a good energy carrier? B. Oxidation-Reduction Reactions Fig. 5.9 p. 122 End of Chapter ‘Study Questions’ Multiple Choice #1 Provide energy for ATP generation Removal and Gain of 6 1. Oxidation 2. Reduction 3. Redox 4. Dehydrogenation 5. NAD+ Fig. 5.10 p. 123 7 C. The Generation of ATP: Phosphorylation of ADP to ATP LO/CYU 5-9 When does ‘ADP + P Text p. 122 ATP’ actually occur? 1. Substrate-level phosphorylation 3 ways ATP is formed 2. Oxidative phosphorylation 3. Photophosphorylation End of Chapter ‘Study Questions’ Review #5 8 Substrate-level phosphorylation A chemical reaction where a phosphate group is transferred from a molecule to ADP. This requires a specific enzyme that can transfer this phosphate from this specific molecule (the enzyme’s substrate) to ADP. ATP is produced this way during FERMENTATION Glycolysis (or alternative pathways) Krebs cycle Oxidative phosphorylation Energy is released from transfer of electrons (oxidation) from one compound to another (reduction). This energy is used to generate ATP in the electron transport chain. An electron transport chain (ETC) couples a chemical reaction between an electron donor (such as NADH) and an electron acceptor (such as a cytochrome molecule) to the transfer of H+ ions across a membrane (this transfer of H+ ions is called chemiosmosis). This occurs during a set of biochemical reactions. This occurs during respiration. Photophosphorylation Light causes chlorophyll to give up electrons. The electrons go through a process similar to what happens during respiration (an electron transport chain and chemiosmosis occur). This process releases energy that is used to bond a phosphate to ADP producing ATP. The ATP produced is used to produce food molecules (sugars-glucose). 9 C. Metabolic Pathways of Energy Production LO/CYU 5-10 In the cell, there are many series of enzymatically catalyzed chemical reactions that store energy and release energy from organic molecules- carbohydrates, proteins, and lipids. Catabolic reactions with these molecules release energy for ATP production and anabolic reactions use the energy in those ATPs primarily to synthesize large forms of these molecules. Why do cells use metabolic pathways to release energy? IV. Carbohydrate Metabolism (CATABOLIC METABOLISM) LO/CYU 5-11 A. Glycolysis Biochemical pathway (10 reactions) in which ONE molecule of GLUCOSE is OXIDIZED to form 2 molecules of PYRUVIC ACID. NAD (nicotinamide adenine dinucleotide, an important electron carrier) is reduced ATP is required in the first stage of glycolysis and formed in the later stage of glycolysis Fig. 5.11 Foundation Figure: An Overview of Respiration and Fermentation Fig. 5.12 Outline of the reactions of glycolysis (Slides 1 & 2) 10 B. Alternatives to Glycolysis Text discussion p. 125, 127 1. Pentose Phosphate Pathway: Breaks down 5-C sugars and glucose. Other needed molecules are produced as intermediates,but it only nets 1 ATP. 2. Entner-Doudoroff Pathway: Breaks down glucose w/ some different enzymes. NEXT: Either Cellular Respiration or Fermentation C. Cellular Respiration (Fermentation later) Define respiration: p. 127 1. Aerobic Cellular Respiration a. After glycolysis is the Preparatory Step before the Krebs Cycle 11 c. Krebs cycle LO/CYU 5-13 Fig. 5.13 p. 128 12 d. Electron transport chain/ Chemiosmosis/ADP Phosphorylation Electron transport chain: A series of electron carriers are oxidized (lose/donate electrons) & other electron carriers are reduced (gained electrons) as electrons are passed from one electron carrier to another. NADH and FADH2 will donate their electrons to electron carriers located in the prokaryotic plasma membrane. Eukaryotes- this occurs in the mitochondria. Oxygen is the final electron acceptor in aerobic respiration. Before continuing, turn the page and draw the electron transport chain. Then come back! Info: Some electron carriers pick up one electron, others pick up more than one electron. Some electron carriers carry hydrogen atoms (1e-, 1p+); others ONLY CARRY ELECTRONS (THE PROTON IS SEPARATED FROM THE ELECTRON IN THE H ATOMS). In the electron transport system, FADH2 donates its electrons after NADH. There also is anaerobic respiration where the final electron acceptor is NOT oxygen. Less common & doesn’t produce as much ATP. 13 Electron Transport Chain 14 Chemiosmosis Fig. 5.15 p. 130, Fig. 5.16 p. 131 LO/CYU 5-14 1) Occurs simultaneously with the electron transport chain (they are coupled) to transfer the energy to form ATP from ADP and phosphate (ADP phosphorylation). 2) At certain points along the electron transport chain, the hydrogen atom is ‘split’; the electron and the proton are separated. Remember, some electron carriers carry hydrogen atoms (1e- & 1p+), others only carry e-. So what happens to the protons in the H atoms when an electron carrier only picks up the e-? 3) The protons are pumped out of the cell (through the plasma membrane) & the electron is then passed to other electron carriers. 4) This creates a situation where there are more protons on the external side of the plasma membrane than are on the internal side of the plasma membrane; in other words, a gradient is formed. 5) This gradient creates a force. 6) Chemiosmosis is the process of creating a proton gradient (by movement of protons across the plasma membrane) and the subsequent movement of the protons back into the cell through specific channels through the enzyme ATP synthase. ATP Generation: ADP Phosphorylation 1) Bonding of a phosphate group to ADP to form ATP. 2) The energy required to bond the phosphate to ADP (and that is then stored in the resulting bond) is provided by the movement of protons back into the cell during chemiosmosis. 3) When the protons rush back into the cell (due to the gradient), energy is released. This may cause a CONFORMATIONAL (shape) change in the enzyme ATP synthase. ATP synthase that then catalyzes the reaction: 15 Fig. 5.16 p. 131 ETC and Chemiosmotic Generation of ATP How many ATPs are generated from aerobic respiration through Substrate-Level Phosphorylation only? How many ATPs are generated from aerobic respiration through Substrate-Level and Oxidative Phosphorylation? SLIDE Comparing Eukaryotes and Prokaryotes: Where in the cell do these processes occur? Overview Fig. 5.17 Summary of aerobic respiration p. 133 Overall Summary Reaction of Aerobic Respiration: 16 Anaerobic Respiration. LO/CYU 5-15 Slide What takes the place of oxygen? EXAMPLES: a. Sulfate. In marine sediments this leads to large amounts of sulfate reduction - Sulfate SO42- is converted (reduced) to hydrogen sulfide H2S - which some may be familiar with as the rotten egg smell and black material that can be found just a few centimeters below sediment surfaces. b. Nitrate NO3-. which is converted (reduced) to nitrite NO21-, nitrous oxide N2O, or nitrogen gas N2 in the process. c. Metal ions. For example: Fe+2, Mn+2 d. Carbonate, CO32-. is converted (reduced) to methane, CH4. This is called methanogenesis Very little energy is obtained from methanogenesis and vast amounts of substrate need to be turned over to make a ‘living’. The amount of energy generated varies depending on the electron acceptor (2-36 ATPs). What are the organisms that undergo anaerobic respiration? Primarily live in conditions. Aerobic respiration: The final electron acceptor in the electron transport chain is molecular oxygen (O2). VS. Anaerobic respiration: The final electron acceptor in the electron transport chain is not O2 (But it is generally an inorganic molecule). Yields less energy than aerobic respiration because only part of the Krebs cycles operates under anaerobic conditions. 17 D. Fermentation LO/CYU 5-16 FERMENTATION Scientific definition: Releases energy from oxidation of organic molecules Does not use oxygen Does not use the Krebs cycle or ETC Uses an organic molecule (pyruvic acid) as the final electron acceptor to form ‘end-products’ (acids and alcohols) 2 ATPs netted Fig. 5.18 b p. 134 18 Two Primary Categories of Fermentation Fig. 5.19 p. 136 Examples of Types & Importance of Fermentation Table 5.4 p. 137 Table 5.4 p. 137 19 See Table 5.5 Aerobic Respiration, Anaerobic Respiration, and Fermentation Compared p. 137 Fig. 5.11 p. 125 Foundation Figure An Overview of Respiration and Fermentation End of Chapter ‘Study Questions’ Review #4 a and b Multiple Choice #7-10 20 V. Lipid and Protein Catabolism LO/CYU 5-17 Summary of the interrelationships of carbohydrate, lipid and protein catabolism Fig. 5.21 p. 138 21 VI. Biochemical Tests and Bacterial Identification: Directly relates to your ‘Unknown’ laboratory exercises/ Not on lecture exam VII. Photosynthesis p. 140-1 CYU 5-19, 5-20, 5-21 http://www.solarnavigator.net/photosynthesis.htm Conversion of light energy into chemical energy (ATP) which is used to synthesize nutrients (glucose) Overall Summary Reaction? Compare and Contrast: Oxidative Phosphorylation and Photophosphorylation. Oxygenic versus Anoxygenic Oxygenic 6 CO2 + 12 H2O + Light energy C6H12O6 + 6 H2O + 6 O2 Anoxygenic 6 CO2 + 12 H2S + Light energy C6H12O6 + 6 H2O + 12 S 22 VIII. A Summary of Energy Production Mechanisms p. 141 LO/CYU 5-22 See Requirements for ATP (energy sources, electron carriers, final electron acceptors) Fig. 5.27 p. 143 End of Chapter ‘Study Questions; Multiple Choice #3 IX. Metabolic Diversity among Organisms p. 142 LO/CYU 5-23 Four Categories (Nutritional Patterns): Where do you get your energy and where do you get your Carbon for the organic molecules you need? Nutritional Type Energy Source Carbon Source Example Photoautotroph Light CO2 Oxygenic: Cya Anoxygenic: G Photoheterotroph Light Organic compounds Green, purple Chemoautotroph Chemical CO2 Iron-oxidizing Chemoheterotroph Chemical Organic compounds Fermentative Animals, proto (See Fig. 5.28 p. 143) End of Chapter ‘Study Questions’ Review #7 23 X. Metabolic Pathways of Energy Use p. 146 LO/CYU 5-24 Now you have ATP and Carbon (and some other basic stuff), what are you going to do with it? Describe the major types of anabolism and their relationship to catabolism. A. Polysaccharide Biosynthesis B. Lipid Biosynthesis C. Amino acid and Protein Biosynthesis D. Purine and Pyrimidine Nitrogen base Biosynthesis 24 XI. The Integration of Metabolism p. 147 LO/CYU 5-25 Amphibolic pathways: Metabolic pathways that have both catabolic and anabolic functions Same figure on Slides 8 & 9. Fig. 5.33 p. 149 25 Metabolism The Big Picture End of Chapter ‘Study Questions’ Review #1h Same figure on Slide 10 26