1.2 learning experience worksheet (doc 113 KB)
advertisement

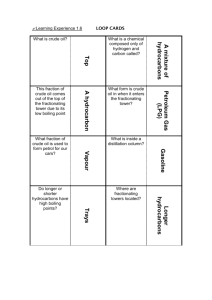
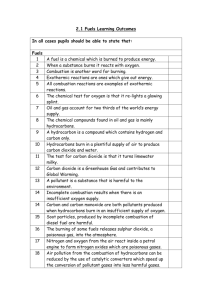
Learning Experience 1.2 Oil is essential to our lives and lifestyles – not just for mobility and heat but for thousands of products which we use or encounter every day. Crude oil is of little use when it first comes out of the ground or from deep below the seabed. It has to be processed and turned into useful products at an oil refinery. Not all crude oils are the same. Some are thick and tarry, for example some from South America, whilst others are lighter with lower density, such as some from the North West Shelf of Australia. How does an oil refinery turn crude oil into the useful products we use every day? Before we learn about the process of fractional distillation it is important to look at what crude oil is actually composed of. Crude oil is a mixture of many different hydrocarbons which vary in length. The larger the hydrocarbon molecule: the the the the the more carbon atoms in its chain higher its boiling point less volatile it is less easily it flows (viscous) less easily it ignites (flammable) In order to turn crude oil into useful products we need to separate the different hydrocarbons into fractions. A particular fraction contains hydrocarbons of similar size, with boiling points in a specific range. For example, fractional distillation produces a ‘gasoline’ fraction which contains hydrocarbons with 8 carbon atoms, and a kerosene fraction which contains hydrocarbons with 15 carbon atoms. ACTIVITY Write definitions for the following terms: hydrocarbon: _____________________________________________________________________ _____________________________________________________________________ fraction: _____________________________________________________________________ _____________________________________________________________________ boiling point: _____________________________________________________________________ _____________________________________________________________________ The process of fractional distillation at an oil refinery Because the various components of crude oil have different boiling temperatures, they can be separated by a process called fractional distillation. The steps of fractional distillation are as follows: 1. You heat the crude oil to a high temperature. 2. The mixture boils, forming vapour (gases); most substances go into the vapor phase. 3. The vapour enters the bottom of a long column (fractional distillation column) that is filled with trays or plates. 1. The trays have many holes or bubble caps (like a loosened cap on a soda bottle) in them to allow the vapor to pass through. 2. The trays increase the contact time between the vapor and the liquids in the column. 3. The trays help to collect liquids that form at various heights in the column. 4. There is a temperature difference across the column (hot at the bottom, cool at the top). 2. The vapour rises in the column. 3. As the vapour rises through the trays in the column, it cools. 4. When a substance in the vapor reaches a height where the temperature of the column is equal to that substance's boiling point, it will condense to form a liquid. (The substance with the lowest boiling point will condense at the highest point in the column; substances with higher boiling points will condense lower in the column.). 5. The trays collect the various liquid fractions. Source: “How Stuff Works” http://science.howstuffworks.com/oil-refining4.htm What must be done to the crude oil before it enters the fractioning tower? _____________________________________________________________________ _____________________________________________________________________ ____________________________ Describe the fractioning tower in terms of temperature. _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ __________________________________________ Do larger or smaller hydrocarbons have a higher boiling point? _____________________________________________________________________ ______________ What type of hydrocarbons turn back into liquids at the bottom of the tower? _____________________________________________________________________ ______________ What happens to the hydrocarbons that have lower boiling points? _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________________________________________ At what point do the very short chained hydrocarbons exit the fractioning tower? _____________________________________________________________________ _____________________________________________________________________ ____________________________