Leg venous ulcers. Results from an ambulatory conservative and
advertisement

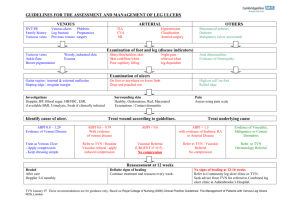
1 Leg venous ulcers. Results from an ambulatory conservative and unchanged treatment of 1.000 consecutive patients during 17 years. A cohort study of ulcer closure. E.G. Bertranou;1 S.E. Gonorazky;1 1 Surgery Division and Department of Research, Hospital Privado de Comunidad de Mar del Plata, Argentina. Email: egbertranou@yahoo.com.ar Objective: This work aims at presenting the results of a venous ulcer cohort study of a 17-year unchanged treatment, based on a conservative, ambulatory approach with compression therapy, in terms of ulcer closure and the risk factors involved (gender, age, ulcer size, diabetes and time between ulcer onset and first visit). Method: This study comprises a cohort of 1.000 consecutive patients diagnosed with venous leg ulcers between 1990 and 2007. The treatment consisted in daily treatment (self care) with silver sulfadiazine ointment, cotton and elastic bandages. Controls for debridement were conducted every fortnight or monthly. To favour actuarial evaluation, ulcers were stratified into three groups based on their size surface area: A ≤10 cm²; B 10–30 cm², and C>30 cm². Kaplan-Meier actuarial analysis and Cox regression technique were applied. Results: Taking into account the healing percentages corresponding to the three surfaces and 4 periods (3, 6, 9 and 12 months), results were as follows: 3 months: A) 62% (95% CI: 58–66%), B) 35% (95% CI: 28–43%) and C) 21% (95% CI: 14–31%); 6 months: A) 81% (95% CI: 78–84%), B) 65% (95% CI: 58–72%) and C) 41% (95% CI: 32–52%); 9 months: A) 87% (95% CI: 84–90%), B) 76% (95% CI: 69–82%) and C) 52% (95% CI: 42–62%); 12 months: A) 90% (95% CI: 87%-92%); B) 80% (95% CI: 74– 86%) and C) 57% (95% CI: 47–67%).The predictor variables of late ulcer closure (risk factors) were ulcer surface, time of ulcer evolution, and patient age. Conclusions: By the end of the sixth month, 81% (95% CI: 78%-84%) of ulcers ≤ 10 cm2 were closed (epithelisation). Ulcer size was the most important risk factor for late closure. This study could help to compare treatment innovations and provide a prognosis for healing time. Keywords: varicose ulcer; leg venous ulcer cohort studies; actuarial analysis; multivariate analysis; outpatient care; risk factors Leg venous ulcers constitute a pathology that seriously affects a person’s quality of life. It is estimated that 1% of the adult population suffers from this type of ulcer in their lifetime, with its prevalence ranging from 0.1% to 0.3% of the population1–3. In 1990 we responded to the challenge of managing several patients with venous leg ulcer with efficacy, good patient compliance/collaboration, easy treatment application, a good cost-effectiveness equation, and a conservative approach. Our first step back in that time was to open an Ulcer Clinic. The numerous and dissimilar treatments suggested in the literature at that time made us rework and extend an assistance program dealing with the objectives mentioned above and, specifically, based on encouraging dressing practice in patients at home. It included wound cleansing, antiseptic ointment use and the application of one layer of soft cotton bandage and one 3 meter long layer of spiral elastic bandage to treat venous insufficiency. Lesion progress was measured at the Ulcer Clinic every 2 to 4 weeks; and the results obtained were reported to the Hospital authorities and to scientific meetings held once a year 4. 2 After our visit to the London Charing Cross Hospital and to the St George’s Hospital, and having read the work by Moffat et al.9, which reported the results from 475 patients coming from six community clinics from the London area treated as determined by actuarial techniques, we realized that we were in the right track. In Moffat’s words: “Ulcer treatment is rarely based on scientific principles but rather on the market forces determining the healing process, without taking into account their efficiency9.” We decided then to continue our work sustaining that the ointment would not change the final result. More patients were enrolled throughout the cohort study with no changes in the treatment protocol. A definite contrast could be noticed with respect to several others that varied compression bandage as well as the ointment used, applying randomized techniques. 1-19. A review of recent literature, including a meta-analysis of the Cochrane system, highlights a number of research works resulting from double-blind trials on small series of randomised patients. All these studies include compression therapy as their primary treatment, reporting, in their conclusion, either improvement or progress in ulcer closure within a few months10–20. Several novel therapeutic approaches, both physical and/or pharmacological, are being tested as adjuvants to treatment; however, few provide any significant evidence of improved efficacy compared to compression therapy alone1–19. Only one article has demonstrated the effectiveness of its intervention. It was a randomised study conducted with 82 patients on the use of compression alone compared to a combination of compression therapy and ameloginin (a protein of the extracellular matrix protein family), with the group with ameloginin achieving a significant reduction in ulcer size20. Despite this, the generalizability of the method and cost-effectiveness of the approach remain to be ascertained. Encouraged by the fact that elastic compression therapy remains the gold standard for leg venous ulcer, we ran the same program for several years in order to assess the treatment in the long run, and to evaluate the risk factors associated with healing. This paper aims at presenting the results obtained from treating 1.000 patients with venous leg ulcers with compression therapy between 1990 and 2007. Despite the large number of patients and long research period, the treatment protocol remained unchanged, with no new therapeutic approaches adopted during the course of the study. Moreover, care was provided by the same staff and in the same clinic throughout the research period. This allowed us to accurately estimate healing time, as well as the risk factors connected to this pathology (gender, age, TUE=time of ulcer evolution or ulcer age, diabetes, etc.) based on the supposition that our cohort was representative of the general population with leg venous ulcer. Methods The study was conducted in the Phlebology Service of the Hospital Privado de Comunidad de Mar del Plata, Argentina, between 1990 and 2007. Inclusion criteria: One-thousand consecutive patients suffering from leg venous ulcer resulting either from superficial and/or deep vein insufficiency whether congenital or after a deep vein thrombosis were enrolled every month in the study. Patients were included irrespective of their ulcer size, location, depth, and swelling and of the healthy appearance of the wound. Exclusion criteria: Insufficient peripheral arterial perfusion. Whenever possible, ABPI had to be > 70 mmHg. For some patients, the test was impracticable (peripheral edema, huge ulcer involving areas of peripheral arteries, painful ulcer, reluctant patient, etc.). In those cases we resorted to portable ultrasound equipment or to toe capillary filling (less than 5 seconds after 5 seconds of compression). The rationale behind this criteria was to have a foot arterial pressure greater than the bandage pressure in the foot and ankle (60 mmHg measured by sphyngomanometer) to avoid arterial 3 compression and to facilitate venous return. If patients tolerated these parameters with their leg horizontal or when walking, the treatment was considered efficient. Otherwise, the bandage pressure had to be adequately fixed. Age, gender, ulcer age and diabetes were not exclusion criteria. Ulcer size was calculated by multiplying the two maximum perpendicular diameters6. The Hospital’s Ethics Committee did not evaluate this study for considering that the treatment was standard. The programme was assessed on a periodic basis and the first results were published in 19924. Treatment Protocol The conservative ambulatory treatment protocol was based on educating patients and families, and started with a long training session on self-care and compression bandage given by trained nurses. The aim of such training was to teach patients how to apply the bandage. A brochure with key information and advice on eating without salt (to avoid peripheral edema in inactive patients) and taking regular walks, among others, was handed to the patients/family. Education is a never-ending task. Treatment was initiated after the clinical diagnosis of venous leg ulcer and after verifying the inclusion and exclusion criteria above specified. For control purposes, ulcers were photographed in the initial assessment visit and after their complete closure, and evidence was stored in a database. Deep ulcer debridement was performed under local anaesthetics. This procedure was mostly conducted by specialized nurses at the beginning of the treatment in the Ulcer Clinic, and comprised the removal of necrotic tissues with the aid of scissors and a scalpel. Mechanical debridement followed, and it was achieved with cotton gauze embedded in a hydrophilic antiseptic ointment, and wrapped with an elastic bandage. The gauze pressed on the ulcer producing such debridement when it was removed the day after. Wound care comprised applying moisturizing cream around the ulcer, followed by silver sulfadiazine-lidocaine ointment of hydrophilic composition. Some controversies exist regarding the use of silver sulfadiazine in wounds. The Cochrane Database of Systematic Reviews of January 2006 (Bergin SM and Wraight P) concludes that “Despite the widespread use of dressings and topical agents containing silver for the treatment of diabetic foot ulcers, no randomised trials or controlled clinical trials exist that evaluate their clinical effectiveness”. Indeed, trials are needed to determine clinical benefits and cost-effectiveness as well as long term outcome, including adverse events. Throughout the study, we used Platsul® with silver sufadiazide 10 mg., dimecaine 6.66 mg., and vitamin A 2.5 mg. in a basic hydrophilic ointment. No intolerance or allergy was ever reported by our patients. As postulated before, the ointment is not what matters but rather the way in which the dressing is applied. After that, sterile cotton gauze was placed over the wound and a cotton bandage was loosely wrapped down from the foot to below the knee to prevent the elastic bandage from hurting or irritating the leg skin. The elastic bandage was applied starting from the forefoot (living the heel free) following with the ankle and finishing below the knee. Pressure in the forefoot and ankle was approximately 60 mmHg, so as not to hinder arterial circulation, 40 mmHg at the mid leg segment, and 20 mmHg below the knee, thereby favouring venous return through the perforators (the saphenous and posterior arch veins) toward the deep veins. The service used a device with an inflatable rubber end, calibrated with a mercury manometer, to measure the approximate bandage pressure. The dressing was changed by the patient/family on a daily basis. 4 Curiously leg venous ulcers are not extremely painful unless they are grossly infected. Pain can be efficiently controlled with Ibuprofen and/or Tramadol. A well wrapped elastic bandage is the best analgesic, because it controls the pain of deep venous thrombosis. If a leg venous ulcer is painful in an unreasonable way, an arteriolar compromise could be involved, such as in the Martorell’s arteriolar hypertensive ulcer7. The second visit was scheduled 1 week after treatment initiation, where errors in dressing structure were corrected, such as pressure in the elastic bandage (greater below the knee than in the foot), incorrect use of the ointment, insufficient or to tight cotton bandage, etc. If the pressure in the bandage was not correct, a manometer was provided to the patient/family until the technique was properly mastered. Surgical debridement was performed (if necessary) under topical anesthetics, and an appointment was scheduled for follow-up every 2 weeks or monthly, until full epithelisation took place. Once complete ulcer healing was attained, the surgical treatment of the superficial and/or deep venous insufficiency was addressed, whenever possible. Throughout evolution, data were stored in the database. The primary endpoint of the study was the full epithelisation of the ulcer. The independent variables taken into account were: Ulcer size on initial assessment, stratified into three groups (A ≤10cm²; B 10– 30cm², and C >30cm²) TUE=Time of ulcer evolution (period between ulcer onset and first visit). Age Gender Diabetes We believe that patient’s mobility is not a major risk factor in delayed healing per se. Besides it is very difficult to evaluate for being extremely subjective, and hence it was not include it in the analysis. If a patient presented ulcers on both lower limbs, the largest one, or the one that had appeared first, was analysed. If relapse occurred, only the primary ulcer was analysed. Statistical Analysis Descriptive: For categorial variables, absolute and relative frequencies were used. Regarding intervalic variables, median, interquartile range and range were adopted, because distribution was abnormal (Table 1). Predictor variables of ulcer closure were ascertained from a Cox’s8 proportional-hazards regression model in the univariate and multivariate analysis. In the univariate analysis, variables were considered statistically significant at a 0.15 level, and, in these cases, they were included in the multivariate analysis. The multivariate analysis was conducted using the stepwise method of conditional regression and the significance level used was P≤0,05. A Relative Risk (RR) < 1 meant late closure (Table 2). The probability of ulcer closure was assessed based on ulcer size, stratified in 3 groups. In the 1st, 3rd, 6th and 12th month after the beginning of treatment, the percentage of ulcer closure was estimated with a confidence interval of 95%, following the method by Kaplan and Meier7. The analysis was conducted using the SPSS statistical package (V11.5.1). 5 Results The study period analysed (1990–2007) allowed us to include a total of 1.000 patients. Median age was 70 years (24–95 years). Sixty-five per cent were women, and the median ulcer surface area was of 4.7cm² (range 0.5–942.4 cm²). Thirteen per cent had diabetes, and the median time of ulcer evolution (TUE) was 3 months (0.5–486 months) (Table1) The statistically significant independent variables (risk factors) for ulcer healing were: Age TUE Ulcer size. These three variables were noted to prolong healing time in a directly proportional way being ulcer size the most relevant clinical feature, with a relative risk (RR) in the multivariate analysis of 0,66 (95% CI: 0.59-0,75) (Table 2). Ulcers were separated into three groups based on their surface area: A: <10cm²; B: > 10 < 30cm², and C: > 30cm² (66%, 20% and 14% of patients, respectively). The percentage of healed patients in the different periods in the three stratified groups, based on ulcer size, are shown in Table 3 and Figure 1. Discussion Despite the large number of patients and the prolonged follow-up, which characterised our study, the treatment protocol remained unchanged throughout the series, with no new therapeutic approaches adopted during the course of the study. On the other hand, care was provided by the same staff and in the same clinic throughout the research period. Finally the 1.000 patients were divided into 3 groups based on the ulcer size with the purpose of determining the relevance of this risk factor. We believe that we are in a position to present a basic work based on compression bandage as the gold standard and with no need to include further elements. This research could work as a point of reference for other researches being conducted by adding innovation in small series and improving the treatment of this devastating condition. Additionally, based on this study, a surgeon faced with a venous ulcer could inform a patient/family just by looking at the three curves, “Your ulcer is less than 10 cm2, then it has between 78% and 84% chances of being closed after 6 months”, for instance (Figure 1). The results obtained from this series can be compared to those of similar works including a large number of patients (multi-centric studies) and with ulcer closure as their primary outcome21,22. Two articles have demonstrated the action of purified micronised flavonoid fraction21,22. Glinski et al.21 conducted a double-blind study, carried out on 140 patients with venous leg ulcers of area >3cm², treated for 6 months. Patients treated with the purified micronised flavonoid fraction achieved a 71% closure, compared to our series result of 80% closure for ulcers of area >10cm², during the same period (Figure 1). The second work, by Guilhou et al.,22 performed on 107 patients with ulcer area <10cm², achieved 32% closure after 2 months of treatment using purified micronised flavonoid fraction, compared to 662 patients with ulcers of up to 10cm², achieving a 40% closure rate with compression therapy during that same period in our study (Figure 1). 6 Nelson E A and Jones J20 found 80 systematic reviews, RCTs and observational studies and performed a grade evaluation of quality of evidence for interventions. They noticed an immense variety of adjuvant treatment which made it very difficult to compare treatment results. This demonstrates that there is sufficient scientific evidence supporting the recommendation for the use of a hemodynamic method, carried out by professionals versed in vascular pathophysiology, based on compression bandage (saphenous and posterior arch veins and perforators) with elastic bandage for venous leg ulcer treatment. It can also be concluded that, while there are several products available for the healing of venous leg ulcers, the scientific evidence justifying their use is weak, probably because the series include only a small number of patients. Finally, adjuvants appear to do little more than increase the cost-benefit equation. Treatment cost and the high frequency of relapse is worth analysing in this type of ulcers. Conclusions The outcome of the complete healing (epithelisation) considering the largest group of ulcers (≤ 10 cm2) is as follows: a) by the end of the 3rd month, 62% (95% CI: 58% 66%); by the end of the 6th month 81% (95% CI: 78%-84%); by the end of the 9th month 87% (95% CI: 84% -90%); and by the end of the 12th month 90% (CI 87%92%). The multivariate analysis shows that ulcers size, TUE and patient’s age are statistically significant predictors of ulcer closure time, with ulcer size being the most important clinical factor. This study could serve as a platform to compare innovations in ulcer treatment and to offer a prognosis of healing time. References 1 2 3 4 5 6 7 8 9 Nelzén, O. Prevalence of venous leg ulcer the importance of the data collection method. Phlebolymphology. 2008; 15: 4, 143–150. Rodriguez-Piñero, M. Epidemiología, repercusión sociosanitaria y etiopatogenia de las úlceras vasculares [in Spanish]. Angiología. 2003; 55: 3, 260–267. Margolis, D.J., Bilker, W., Santannab, J., Baumgartenc, M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002; 46: 381–386. Bertranou, E.G. Reorganization of phlebology in a general hospital. In: Prescott, R., Raymond-Martinbeau, P. (eds). Phlebology. John Libbey Eurotext Ltd, 1992. Moffat, C.J., Franks, P.J., Oldroy, M. et al. Community clinics for leg ulcer and impact on healing. BMJ. 1992; 305: 6866, 1389–1392. Stacey, M.C., Burnand, K.G., Layer, G.T. et al. Measurements of the healing of venous ulcers. Aust NZ J Surg. 1991; 61: 11, 844–888. Bertranou, E.G., Gonorazky S.G., Otero, A..E. Martorell’s arteriolar hypertensive leg ulcer: results of ambulatory treatment on 365 cases. Phlebology 2001;54:267-272. Cox, D.R., Snell, E.J. The Analysis of Binary Data (2nd edition). Chapman and Hall, 1989. Kaplan, E.L., Meier, P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958; 53: 457–460. 7 10 Senet, P., Bon, F.X., Benbunam, M. et al. Randomized trial and local biological of autologous platelets used as adjuvant therapy for chronic venous ulcers. J Vasc Surg. 2003; 38: 6, 1342–1348. 11 Kopera, D., Kokol, R., Berger, C., Haas, J. Does the use of low-level laser influence wound healing in chronic venous leg ulcer? J Wound Care. 2005; 14: 8, 391–394. 12 Nelson, E.A., Mani, R., Vowden, K. Intermittent compression for treating venous leg ulcers C. Cochrane Database Syst. Rev. 2008; 16: 2, CD001899. 13 O’Meara, S., Al-Kurdi, D., Ovington, L.G. Antibiotics and antiseptics for venous leg ulcer. Cochrane Database Syst. Rev. 2008; 23: 1, CD003557. 14 Kranke, P., Bennett, M., Roeckl-Wiedmann, I., Debus, S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst. Rev. 2004; 2: CD004123. 15 Jull, A.B., Waters, J., Arroll, B. Pentoxifylline for treating venous ulcers. Cochrane Database Syst. Rev. 2002; 1: CD001763. 16 Al-Kurdi, D., Bell-Syer, S.E., Flemming, K. Therapeutic ultrasound for venous leg ulcer. Cochrane Database Syst. Rev. 2008; 3: 1, CD001180. 17 Jull, A.B., Rodgers, A., Walker, N. Honey as topical treatment for wounds. Cochrane Database Syst. Rev. 2008; 8: 4, CD005083. 18 Palfreyman, S.J., Nelson, E.A., Lochiel, R., Michaels, J.A. Dressing for healing venous leg ulcers. Cochrane Database Syst. Rev. 2006; 19: 3, CD001103. 19 Sánchez-Vázquez, R., Briceño-Rodríguez, G., Cardona-Muñoz, E.G. et al. Isosorbide dinitrate spray as therapeutic strategy for treatment of chronic venous ulcer. Angiology. 2008; 59: 1, 64–71. 20 Vowden, P., Romanelli, M., Price, P. Effect of amelogenin extracellular matrix protein and compression on hard-to-heal venous leg ulcer. J Wound Care. 2007; 16: 5, 189–195. 21 Glinski, W., Chodynicka, B., Roszkiewicz, J. et al. Effectiveness of a micronized purified flavonoid fraction (MPFF) in the healing process of lower limb ulcers. An open multicenter study, controlled and randomized. Minerva Cardioangiol. 2001; 49: 2, 107–114. 22 Guilhou, J.J., Dereure, O., Marzin, L. et al. Efficacy of Daflon 500mg in venous leg ulcer healing: A double-blind, randomized, controlled versus placebo trial en 107 patients. Angiology. 1977; 48: 1, 77–85. 23 Nelson, EA, Jones J. Venous leg ulcer. Clin Evid (On line). 2008 Sep. 15, 2008. 8 Table 1 Number of Patients: Females: Age of females (in years): Males: Age of males: Ulcer size: TUE Diabetes: Follow-up: 1.000 653 (65,3%) 71 years (M); 63 - 77 years (IR); 32 - 95 years 347 (34,7%) 69 years (M); 61 - 75 years (IR); 24 - 93 years (R) 4,7 cm² (M); 1,6 - 14,1 cm² (RI); 0,5 - 942,4 cm² (R) 3 months (M); 1-3 months (IR) 1- 486 (R) 128 patients (12,8 %) 2,3 months (M); 1-5 months (IR); 0-58,2 months (R) M: Median IR: Interquartile range R: Range TUE: Time of ulcer evolution (Period between ulcer onset and first visit) Table 2 Predictor variables of ulcer closure (RR*<1 late closure) Univariate Analysis Factors Age Gender ‡ Ulcer size § TUE ¶ Diabetes RR* 0,99 1,03 0,60 0,99 0,86 (IC 95%)† (0,99-1) (0,89-1,2) (0,54-0,67) (0,98-0,99) (0,69-1,06) Multivariate Analysis p 0,08 0,18 & <0,05 <0,05 0,16 & RR* 0,99 ---0,66 0,99 ---- (IC 95%)† (0,99-1) ----(0.59-0,75) (0,990-0,997) ---- * RR= Relative risk † 95% CI = 95% Confidence limits ‡ Gender=male & Not significant § Ulcer size divided into three groups: A ≤10 cm 2; B 10-30 cm2: C >30 cm2 ¶ TUE=time of ulcer evolution (period between ulcer onset and first visit) p <0,05 ----< 0,05 <0,05 ---- 9 Table 3 Percentage of healed patients in different periods in the three stratified groups, based on ulcer size. Months Group A ≤10cm² n=662 Group B 10–30cm² n=196 Group C >30cm² n=142 1 15% (12%; 18%)* 2% (1%; 6%)* 3% (1%; 8 %)* 3 62% (58%; 66%)* 35% (28%; 43%)* 21% (14%; 31%)* 6 81% (78%; 84%)* 65% (58%; 72%)* 41% (32%; 52%)* 9 87% (84%; 90%)* 76% (69%; 82%)* 52% (42%; 62%)* 12 90% (87%; 92%)* 80% (74%; 86%)* 57% (47%; 67%)* * 95% confidence interval Fig 1. Actuarial analysis conducted on 1000 patients 10