DEAE Dex Transfection Protocol
advertisement
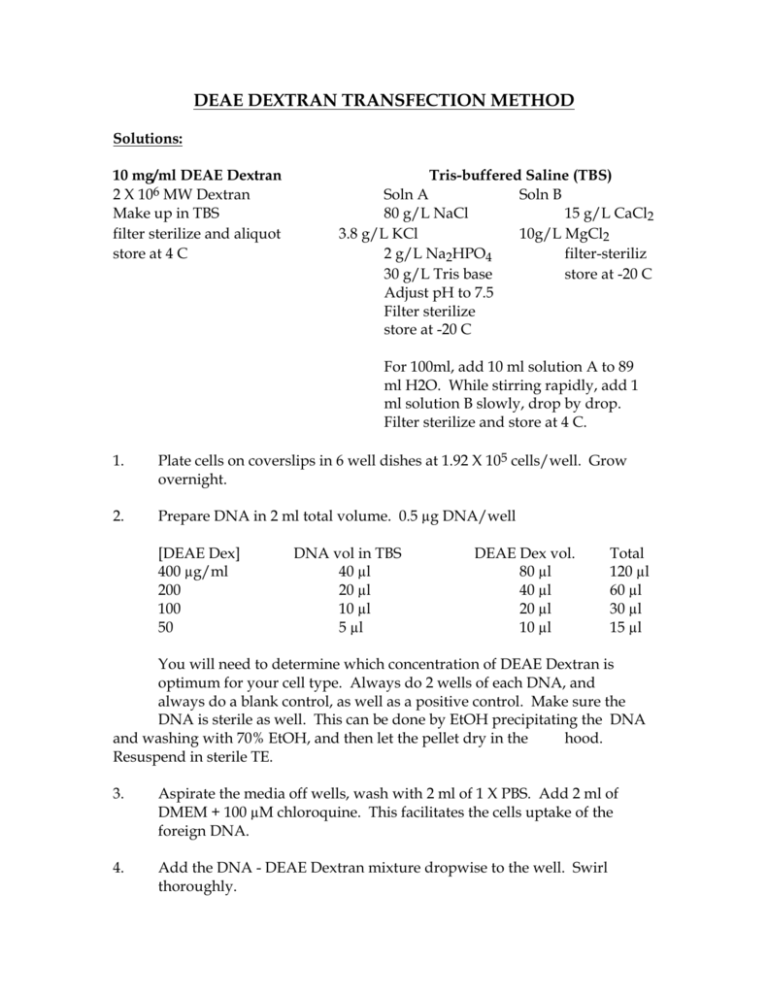
DEAE DEXTRAN TRANSFECTION METHOD Solutions: 10 mg/ml DEAE Dextran 2 X 106 MW Dextran Make up in TBS filter sterilize and aliquot store at 4 C Tris-buffered Saline (TBS) Soln A Soln B 80 g/L NaCl 15 g/L CaCl2 3.8 g/L KCl 10g/L MgCl2 2 g/L Na2HPO4 filter-steriliz 30 g/L Tris base store at -20 C Adjust pH to 7.5 Filter sterilize store at -20 C For 100ml, add 10 ml solution A to 89 ml H2O. While stirring rapidly, add 1 ml solution B slowly, drop by drop. Filter sterilize and store at 4 C. 1. Plate cells on coverslips in 6 well dishes at 1.92 X 105 cells/well. Grow overnight. 2. Prepare DNA in 2 ml total volume. 0.5 µg DNA/well [DEAE Dex] 400 µg/ml 200 100 50 DNA vol in TBS 40 µl 20 µl 10 µl 5 µl DEAE Dex vol. 80 µl 40 µl 20 µl 10 µl Total 120 µl 60 µl 30 µl 15 µl You will need to determine which concentration of DEAE Dextran is optimum for your cell type. Always do 2 wells of each DNA, and always do a blank control, as well as a positive control. Make sure the DNA is sterile as well. This can be done by EtOH precipitating the DNA and washing with 70% EtOH, and then let the pellet dry in the hood. Resuspend in sterile TE. 3. Aspirate the media off wells, wash with 2 ml of 1 X PBS. Add 2 ml of DMEM + 100 µM chloroquine. This facilitates the cells uptake of the foreign DNA. 4. Add the DNA - DEAE Dextran mixture dropwise to the well. Swirl thoroughly. 5. Incubate wells 4 hours at 37 C. 6. Aspirate media 7. Shock cells with 2 ml of 10% DMSO in 1 X PBS. Incubate for 1 minute at RT. Add 10 ml of 1 X PBS to dilute, then aspirate. Wash well with 3 ml of 1 X PBS. Add 2 ml DMEM to well. 8. Grow several days, fix coverslips and immunostain. DEAE Dextran Concentration for several different cell types: L Cells Cos Cells 200 µg DEAE Dextran concentration 400 µg DEAE Dextran concentration









