Phys 282 EXPERIMENT 5
advertisement

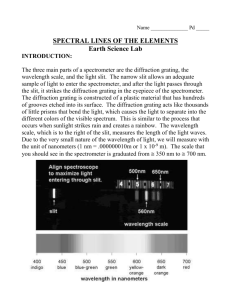
EXPERIMENT 5 SPECTROSCOPY OF LIGHT Print report page to start your lab report OBJECT: To find the wavelengths of the emission lines from different sources. APPARATUS: Mercury light source; diffraction grating; spectrometers; hydrogen and other emission sources. THEORY: In a previous laboratory, the wavelength of the red light from the He-Ne laser was measured by observing the diffraction pattern of the red light after it had passed through a double slit. If more slits are added the diffraction maxima become narrower, but remain at the same angular positions. One benefit of this property is that if two colors of light pass through the multiple slit, aperture (called a diffraction grating), then the two colors produce separate an, distinct diffraction patterns at measurably different angles. It is found that the angular diffraction of light of wavelength by a diffraction grating of slit spacing d obeys the relation n = d sin n where n is the diffraction order number, and n is the angular position of the n-th diffraction maximum. The set of emission colors from elements and chemical compounds are unique signatures of those elements. The measurement of emission wavelengths can allow the identification of elements and compounds present in the light source, whether that source is a distance galaxy or small piece of cloth from the Shroud of Turin. Measurement of the wavelength of these emission colors also provided the initial clues to the atomic structure of the elements. Please read carefully sections on diffraction gratings and atomic spectra in your physics text book (Tipler). The Rydberg-Ritz formula establishes relationship between the emission wavelength and the quantum numbers of initial and final states for the electron: 1/R (1/n22 - 1/n12) , (2) where R is the Rydberg constant, and n1 and n2 are integer numbers (quantum numbers). For the Balmer series (in the visible part of spectrum) n2= 2, and n1> n2 (starting from the red line). PROCEDURE Exercise 1. Obtain a transmission diffraction grating and a mercury light source. Assemble them as shown on Fig. 1. By looking through the grating towards the light source, locate the first position of the virtual image along the transverse meter stick (screen). Figure 1. Sketch of the diffraction grating spectrometer used to measure the wavelength of light. Figure 2 This will be the location of the first order diffraction maximum for that particular color. Measure the grating to screen distance, L. Measure the diffraction image distance, x, as shown in Figure 2, for each emission line. Exercise 2. Take the grating spectrometer that is supplied, and record the positions of mercury emission lines xs. Exercise 3. Measure the positions of all four emission lines from the hydrogen lamp using the spectrometer from Exercise 2. Exercise 4. Repeat the Exercise 3 for the helium and neon sources. DATA ANALYSIS Exercise 1. Calculate the first diffraction angle 1 of each color of light emitted by the light source tan x / L. (3) Calculate the wavelengths of all emission lines using equation (1). Compare your results with the published wavelengths for the mercury source. Exercise 2. Plot the wavelengths obtained in Exercise 1 as a function of xs . This will give you a linear graph. This is the calibration curve of the spectrometer. Exercise 3. Using calibration curve from Exercise 2 find the wavelengths for the hydrogen emission lines. Plot the reciprocal wavelengths as function of 1/n12. Fit the line to the Rydberg-Ritz formula (2) and find R. Compare your R with the published result. Exercise 4. Using the calibration curve for the spectrometer find the helium and neon emission wavelengths. Compare your results with the published values.