LAB: The Synthesis of Table Salt

The Synthesis of Table Salt
One experiment and one report per person.
The Report
Use standard headings for lab reports. Show all data and calculations, and lay them out neatly and attractively. Answer the question posed at the end of the lab.
The Problem
Chemical arithmetic is important to industry. Production of chemicals is measured in millions of tonnes each year and calculation errors would be very costly. In today’s experiment you will not only make salt, but you will be able to predict – to within 0.01 g – how much table salt will be produced. That’s amazing!
The balanced equation for today’s experiment is
HCl
(aq)
+ NaHCO
3 (s)
NaCl
(aq)
+ CO
2 (g)
+ H
2
O
(l)
Reagents and Equipment
sodium hydrogencarbonate powder (NaHCO
3 (s)
)
3 M hydrochloric acid solution (HCl
(aq)
) large test tube (20 cm in length) acid resistant (silica) boiling chips test tube holder or clamp medium sized beaker (about 200 mL)
10 mL graduated cylinder
Bunsen burner
CAUTION: Acids can burn skin. If any spills on you, wash immediately with cold water. The fumes are noxious – use good ventilation and avoid breathing them.
Data
mass of large test tube and boiling chips _______ g mass of NaHCO
3 (s)
_______ g mass of test tube, boiling chips and product _______ g
Procedure
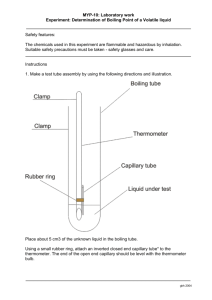
1.
Get a large test tube. Ensure it is clean and DRY. Wash it and dry it over a
Bunsen burner if necessary.
2.
Place two boiling chips into the test tube. These will assist later by allowing smooth boiling. They are not part of the reaction.
3.
Place the test tube and boiling chips onto the scale. To do this, place a small beaker on the scale and push the “Tare” or “Zero” button. (Be careful not to push the wrong button, or you may change the units from grams to ounces.) Place the test tube into the beaker, and record the combined mass of the test tube and boiling chips.
“The Synthesis of Table Salt” Revised January 30, 2004
Continued over …
4.
Without removing the test tube from the scale, zero the scale again. Then measure
1.5 – 2 g of NaHCO
3 (s)
into the test tube. Record the mass to two decimal places.
5.
Measure about 10 mL of 3M HCl
(aq)
into a 10 mL graduated cylinder.
6.
Very carefully and slowly, pour the HCl
(aq)
into the test tube. There will be lots of frothing and foaming. Keep adding HCl
(aq)
until the bubbling stops. At this point the reaction is complete and all the NaHCO
3 (s)
has been consumed. There will be some unreacted HCl
(aq)
in the test tube. This is fine.
7.
Heat the test tube carefully over a Bunsen burner to boil off the excess HCl
(aq)
and water.
You may do this in the fume hood. (The fumes bother some people more than others – work where you like.) In any case, avoid breathing the vapours during boiling, unless you have a real urge to clean out your sinuses. Boil until you are left with a dry, white solid.
Caution - Ensure the mouth of the test tube is pointed away from yourself and any one else .
8.
When cool enough, find the mass of the test tube and contents. The NaCl
(s)
may be kept, sold to a grocery store, or washed down the sink. Don’t eat it, though, because of trace amounts of HCl
(aq)
.
Calculations
1.
Subtract the mass of the test tube and boiling chips from the mass of the test tube, boiling chips, and product. This is the mass of NaCl
(s) produced and is called the experimental yield.
2.
Calculate the number of moles of NaHCO
3 (s)
placed in the test tube.
3.
Using the result from calculation 2 and the mole ratios from the balanced chemical equation, calculate the predicted number of moles of NaCl
(s)
produced.
4.
Convert moles of NaCl
(s)
to mass of NaCl
(s)
. This mass is called the theoretical yield .
5.
How close were your experimental results to the predicted value?
To determine this calculate the percent yield :
experimental yield percent yield = __________________________ x 100%
theoretical yield
Question
1.
a) A reaction occurs between calcium carbonate and hydrochloric acid to produce calcium chloride, carbon dioxide and water. If 3.00 g of calcium carbonate is consumed in the reaction, what mass of calcium chloride is produced? b) If 2.90 g of calcium chloride is actually produced in the experiment described in |
1a),what is the percent yield of CaCl
2
?