Moles & Molar Mass
advertisement
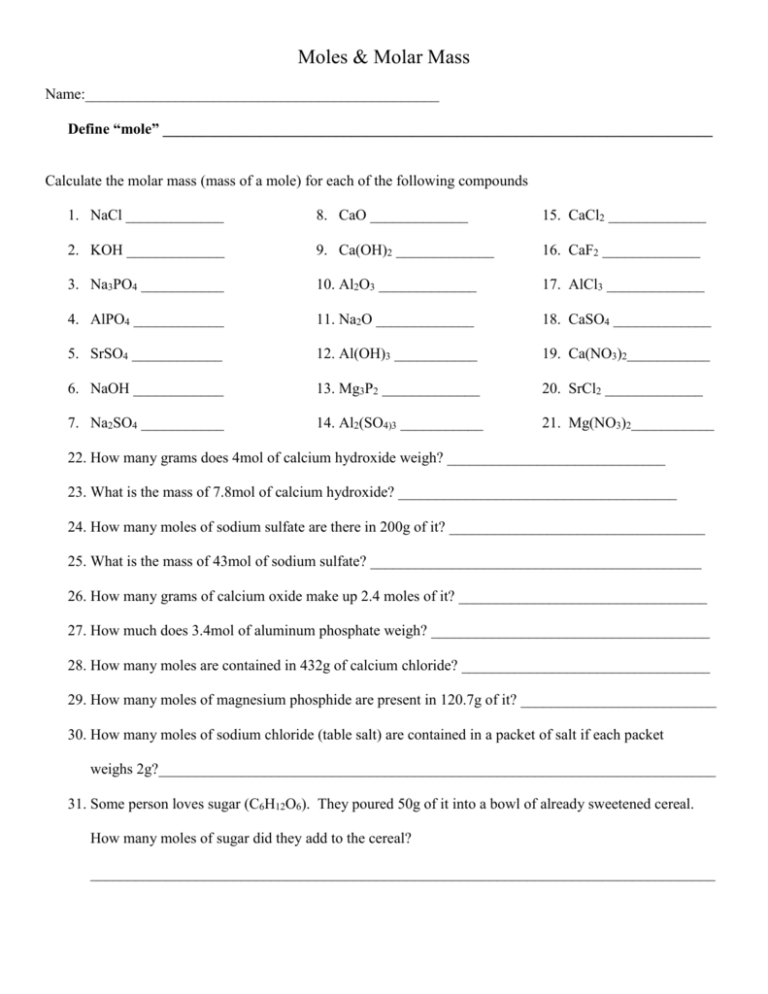
Moles & Molar Mass Name:_______________________________________________ Define “mole” _________________________________________________________________________ Calculate the molar mass (mass of a mole) for each of the following compounds 1. NaCl _____________ 8. CaO _____________ 15. CaCl2 _____________ 2. KOH _____________ 9. Ca(OH)2 _____________ 16. CaF2 _____________ 3. Na3PO4 ___________ 10. Al2O3 _____________ 17. AlCl3 _____________ 4. AlPO4 ____________ 11. Na2O _____________ 18. CaSO4 _____________ 5. SrSO4 ____________ 12. Al(OH)3 ___________ 19. Ca(NO3)2___________ 6. NaOH ____________ 13. Mg3P2 _____________ 20. SrCl2 _____________ 7. Na2SO4 ___________ 14. Al2(SO4)3 ___________ 21. Mg(NO3)2___________ 22. How many grams does 4mol of calcium hydroxide weigh? _____________________________ 23. What is the mass of 7.8mol of calcium hydroxide? _____________________________________ 24. How many moles of sodium sulfate are there in 200g of it? __________________________________ 25. What is the mass of 43mol of sodium sulfate? ____________________________________________ 26. How many grams of calcium oxide make up 2.4 moles of it? _________________________________ 27. How much does 3.4mol of aluminum phosphate weigh? _____________________________________ 28. How many moles are contained in 432g of calcium chloride? _________________________________ 29. How many moles of magnesium phosphide are present in 120.7g of it? __________________________ 30. How many moles of sodium chloride (table salt) are contained in a packet of salt if each packet weighs 2g?__________________________________________________________________________ 31. Some person loves sugar (C6H12O6). They poured 50g of it into a bowl of already sweetened cereal. How many moles of sugar did they add to the cereal? ___________________________________________________________________________________ 32. It is recommended that you eat around 270g of sugar per day. How many moles is this? ____________________________________________________________________________________ 33. How many moles are present in 34 grams of Cu(OH)2? 34. How much does 4.2 moles of Ca(NO3)2 weigh? 35. What is the molar mass of MgO? 36. How are the terms “molar mass” and “atomic mass” different from one another? 37. What is the mass of one mole of Americium? 38. A silicon chip used in an integrated circuit of a microcomputer has a mass of 5.68g. How many moles of silicon are present in this chip? 39. Juglone is a dye with a chemical formula of C10H6O3. Calculate the molar mass of juglone. 40. How many moles are present in 214g of juglone? 41. Calcium carbonate, aka calcite is the principle mineral found in limestone, marble, and chalk. Calculate the molar mass of calcium carbonate. 42. A certain sample of calcium carbonate contains 4.86 moles. What is the mass in grams of this sample?





