Homework 10 Acids and Esters
advertisement

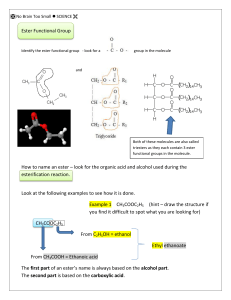
Homework 10 1. Acids and Esters Which of the following compounds is hydrolysed when warmed with sodium hydroxide solution? 2. Propanoic acid is reacted with ethanol, one of the products is, 3. Rum flavouring is based on a compound with the formula shown, It can be made from, A B C D ethanol and butanoic acid propanol and ethanoic acid butanol and methanoic acid propanol and propanoic acid 4. Which of the following is an ester? 5. An ester can be prepared from methanoic acid and ethanol. Which of the following is the full structural formula for the ester produced? 6. Which of the following consumer products is least likely to contain esters? A B C D flavourings perfumes solvents toothpastes 7. Aspirin is one of the most widely used pain relievers in the world. It has the structure, Which two functional groups are present in an aspirin molecule? A B C D 8. hydroxyl and carbonyl aldehyde and ketone carboxyl and ester ester and aldehyde On the structure shown, four hydrogen atoms have been replaced by letters W,X,Y and Z. Which letter corresponds to the hydrogen atom which can ionise most easily in aqueous solution? A B C D 9. W X Y Z Esters are formed when carboxylic acids react with alcohols, (a) Name the ester formed in the above reaction. (1) (b) Name the type of reaction that takes place. (1) (c) To find out which atoms of the carboxylic acid and the alcohol go to form the water molecule, the reaction was carried out using an alcohol in which the 16O atom was substituted by the 18O isotope. All of the 18O was found in the ester and none in the water. Which atoms in the acid and alcohol combine to form the water molecule? (1) 10. Presented below is a simplified flow chart showing some of the processes used in the production of petroleum based consumer products, Processes A to F are contained in the following list; addition polymerisation, catalytic hydration, cracking, fermentation, fractional distillation, hydrolysis, oxidation, reforming. (a) Identify each of the processes A to F. (1) (b) Draw the full structural formula for the product of the esterification step in the flow chart. (1) (c) In the laboratory preparation of an ester, sodium hydrogencarbonate is added at the end of the reaction. (i) (ii) Why is this done? (1) What evidence, apart from the smell, shows that a new substance is formed? (1) 11. One of the chemicals released in a bee sting is an ester that has the structure shown. This ester can be produced by the reaction of an alcohol with an alkanoic acid. (a) Name this acid. (1) (b) The ester can be prepared in the lab by heating a mixture of the reactants with a catalyst. (1) (i) (ii) 12. Name the catalyst used in the reaction (1) What improvement could be made to the experimental setup shown in the above diagram? (1) Synthetic perfumes are cheaper and easier to produce than natural perfumes. Phenylethanol has a smooth rose-like odour and is used in floral perfumes together with its propanoate ester. CH2HC2OH + phenylethanol mass of one mole =122g CH3CH2COOH X + propanoic acid propanoate ester mass of one mole =74g mass of one mole =178g (a) Draw the structural formula for ester X (b) 3.05 tonnes of phenylethanol was refluxed with H2O