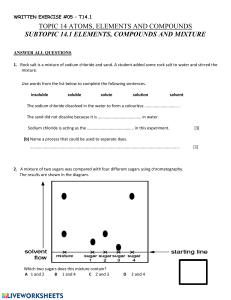
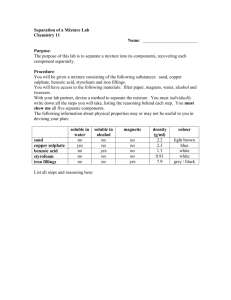
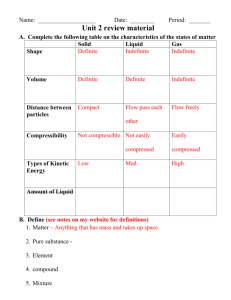
Separation of a Mixture Lab Sheet
advertisement

Lab Report Sheet Name:__________________________ Lab Title: Separation of a mixture. Date:________ Block:________ Name of lab Partner:_______________________________ You are going to separate a mixture today in lab working with a partner. PURPOSE OF LAB: 1. To separate a mixture of sand, sodium chloride, iron filings, and marbles (or some other large object). 2. To determine the mass of each substance. 3. To calculate the percent error comparing total amount of substance to begin with and the amount of substance recovered. Pre-lab Discussion: The following properties of each substance are listed below. You and your partner are to spend some time in discussion and map out a strategy of how each substance will be separated and then how you will determine the mass of each substance. SUBSTANCE Soluble in water? Magnetic? Large or small particles Sand (SiO2) sodium chloride Iron Marbles or other object Equipment: -List any equipment you use in this space. Make sure you use the proper names for the pieces of equipment used. If you need a reference, use the laminated sheet provided by the instructor. Materials: Sample of mixture containing iron, sodium chloride, sand, and pennies, tap water, distilled water. Determine the initial mass of the mixture: ________________________ 1 Draw a qualitative scheme to demonstrate how you separated each component from the mixture. Determine the mass of each component and place the mass next to the final box. Percent Error: 2