Standard Curves with Food Coloring
advertisement
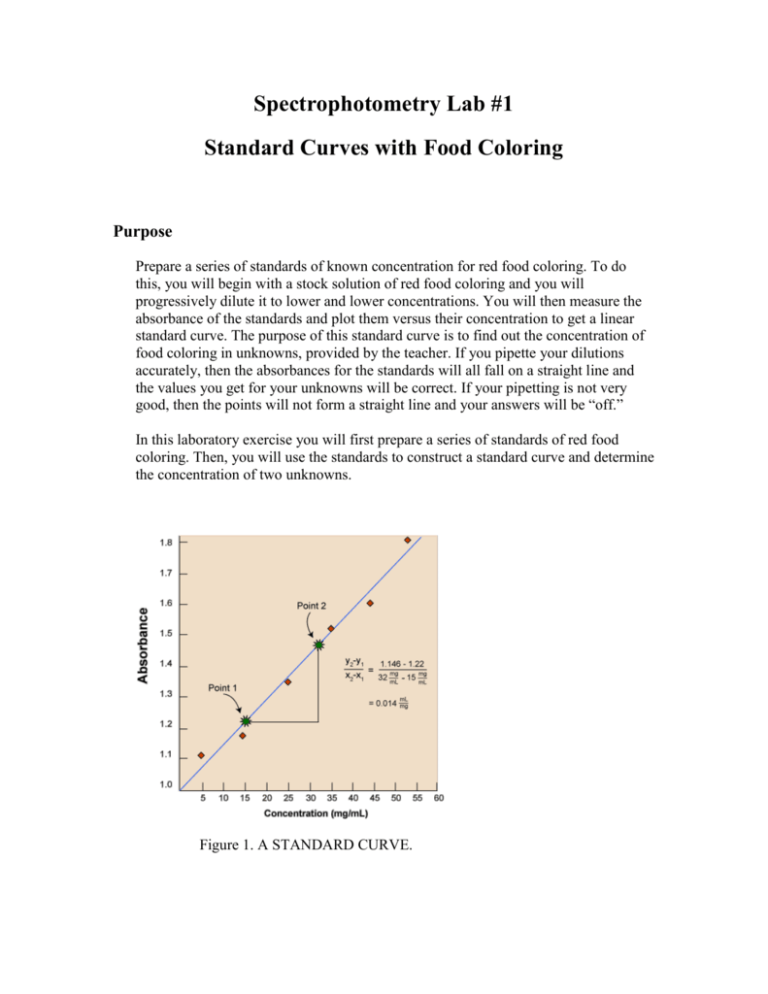
Spectrophotometry Lab #1 Standard Curves with Food Coloring Purpose Prepare a series of standards of known concentration for red food coloring. To do this, you will begin with a stock solution of red food coloring and you will progressively dilute it to lower and lower concentrations. You will then measure the absorbance of the standards and plot them versus their concentration to get a linear standard curve. The purpose of this standard curve is to find out the concentration of food coloring in unknowns, provided by the teacher. If you pipette your dilutions accurately, then the absorbances for the standards will all fall on a straight line and the values you get for your unknowns will be correct. If your pipetting is not very good, then the points will not form a straight line and your answers will be “off.” In this laboratory exercise you will first prepare a series of standards of red food coloring. Then, you will use the standards to construct a standard curve and determine the concentration of two unknowns. Figure 1. A STANDARD CURVE. Laboratory Activities Part A. Dilutions 1. You will be given a sample of red dye at a concentration of 25,000 ppm. 2. What wavelength should you choose to construct a standard curve for red dye? 3. Set your spectrophotometer for the proper wavelength. 4. Check the absorbance of the red dye. ______________________ 5. Oh no! What is the problem here? __________________________ 6. I will tell you that for red food coloring, concentrations in the range of about 1 - 70 ppm will yield absorbance readings that are in the linear range of the spectrophotometer. This means that you must dilute the red dye quite a lot before you can construct a standard curve. There are many dilution strategies. The best one depends on the volumes you need, the glassware you have available, and the pipettes you are using. I will show you one strategy to figure out how to dilute the red dye appropriately. 7. Diluting The Red Dye: (Remember to Mix Well at Each Step!) Prepare 10 ml of 1000 ppm stock from the original sample. You can approach this as a C1V1 = C2V2 problem. mL of original red dye required _______ mL water required _______ Using your 1000 ppm stock, prepare 20 mL of 100 ppm stock. mL of 1000 ppm stock red dye required _______ mL water required _______ Using your 100 ppm stock, prepare 40 mL of 10 ppm stock. mL of 100 ppm stock red dye required _______ mL water required _______ Using your 10 ppm, 100 ppm and 1000 ppm stocks, prepare 10 mL of standards of 4 ppm, 6 ppm, 8 ppm, 10 ppm, 50 ppm, 75 ppm, 100 ppm, and 200 ppm. (We expect some of these to be above the linear range for red food coloring.) It is useful to construct a table, such as shown below before you prepare the final standards STANDARD 1 2 3 4 5 6 7 8 PPM IN STOCK TO ML OF STANDARD USE STOCK REQUIRED 4 6 8 10 50 75 100 200 ML WATER REQUIRED Prepare the standards according to the table you prepared. Check your work. All the standard tube should have the same volume and you should be able to see the color in the standard tubes get progressively darker as the concentration increases. If not, something is wrong 8. Prepare a standard curve. Measure the absorbance for each standard. Graph the data with absorbance on the Y axis and concentration on the X axis and have the graph checked by your instructor. The better your pipetting technique, the closer all your points will be to forming a straight line. 9. Use your standard curve to determine the concentration of food coloring in the three unknowns. If the concentration of food coloring in the unknowns is too high to be read by the spectrophotometer, then dilute them. Calculate the concentration in the original, undiluted sample by multiplying by the dilution factor. Discussion Questions 1. Compare your standard curve and your values for the unknowns with your partner. How close were they? What does this tell you about your pipetting technique? 2. Compare the results you obtained using just 5 standards to those obtained with all 8. Which gives more accurate values for the unknowns? Explain what it means to be in the linear range of the standards. 3. Check the answer key to find out the concentration of red food coloring in the unknown samples. How close were your values for the unknowns to what you expected based on the answer key? Discuss these results. Remember, the whole point of preparing a standard curve is to find the concentrations of analyte in the unknowns. This project is funded by a grant awarded under the President’s Community Based Job Training Grant as implemented by the U.S. Department of Labor’s Employment and Training Administration (CB-15-162-06-60). NCC is an equal opportunity employer and does not discriminate on the following basis: against any individual in the United States, on the basis of race, color, religion, sex, national origin, age disability, political affiliation or belief; and against any beneficiary of programs financially assisted under Title I of the Workforce Investment Act of 1998 (WIA), on the basis of the beneficiary’s citizenship/status as a lawfully admitted immigrant authorized to work in the United States, or his or her participation in any WIA Title I-financially assisted program or activity. “This workforce solution was funded by a grant awarded under the President’s Community-Based Job Training Grants as implemented by the U.S. Department of Labor’s Employment and Training Administration. The solution was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership. This solution is copyrighted by the institution that created it. Internal use by an organization and/or personal use by an individual for non-commercial purposes is permissible. All other uses require the prior authorization of the copyright owner.”




