Reactivity Series of Metals Lab
advertisement

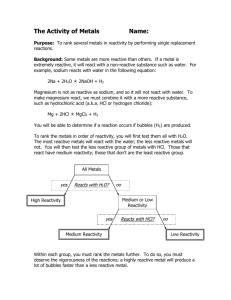
Reactivity Series of Metals Lab Name: ________________________________________ Period: _______________ Purpose: The purpose of this experiment is to determine the order of reactivity (from least reactive to most reactive) of the following six metals: magnesium, copper, zinc, silver, potassium, lead. Hypothesis: Predict what the order will be (from least reactive to most reactive): ____________, _____________, _____________, ____________, _____________, ____________ Procedure: Design a procedure to test the hypothesis using the following solid metals and solutions of metal ions: Solids: magnesium, zinc, copper Solutions: silver nitrate (Ag), copper sulfate (Cu), zinc chloride (Zn), lead nitrate (Pb), and potassium chloride (K) HINTS: If a solid metal is placed in a solution of a different metal, and you observe a chemical reaction, then the solid is more reactive than the metal that is in the solution. If no reaction occurs, then the metal from the solution is more reactive than the solid metal EXAMPLE: Iron metal is placed in nickel nitrate (Ni). You will be observing the reactivity of iron vs. nickel. o If you see a reaction, iron (the solid) is more reactive than nickel (the solution) o If you see no reaction, nickel (the solution) is more reactive than iron (the solid) Required Participation: Behave appropriately in lab, conduct the experiments as instructed, and clean up. You must fill out the data table and get my initials. This lab write-up is a menu option and is worth 15 points—you must get my initials, fill out the data table and results table, write down procedures, and answer the post-lab questions. My initials (1 pt): _____________ Data Table—Required! (4 pts) Use the following table as you need it. You may need all of the squares or not. Be sure to label! Procedures (4 pts) In the following spaces, write as many procedures as are necessary to test the reactivity. You may not use all of the numbers, or if you need more, attach your own sheet of paper. 1. _________________________________________________________________________ _________________________________________________________________________ 2. _________________________________________________________________________ _________________________________________________________________________ 3. _________________________________________________________________________ _________________________________________________________________________ 4. _________________________________________________________________________ _________________________________________________________________________ 5. _________________________________________________________________________ _________________________________________________________________________ Results Table (5 pts) Least reactive Most reactive Post-Lab Questions (1 pt) You must answer this on your own—you may use only notes and your packet to help you. 1. Explain how you determined the order that you have posted in your results table. Did your procedures work? ________________________________________________________________________________ ________________________________________________________________________________