File - MR. POULIN`S NOTES
advertisement

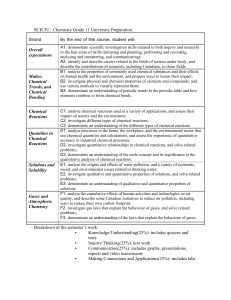
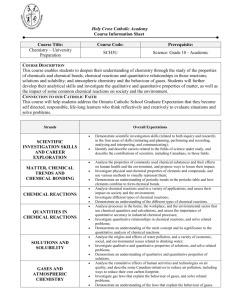
Almonte & District High School Grade 11 University Chemistry Course Code: SCH 3U1 Prerequisite: Science, Grade 10, Academic Reference: The Ontario Curriculum Grades 11 & 12: Science 2008 Textbook: Nelson Chemistry 11 Materials Needed: graph paper scientific calculator Teacher: Mr. N. Poulin Email: nick.poulin@ucdsb.on.ca (Replacement cost $105, rebinding cost $10) lab notebook ruler regular class materials Course Description This course enables students to deepen their understanding of chemistry through the study of the properties of chemicals and chemical bonds; chemical reactions and quantitative relationships in those reactions; solutions and solubility; and atmospheric chemistry and the behaviour of gases. Students will further develop their analytical skills and investigate the qualitative and quantitative properties of matter, as well as the impact of some common chemical reactions on society and the environment. Overall Curriculum Expectations: Matter, Chemical Trends, and Chemical Bonding analyse the properties of commonly used chemical substances and their effects on human health and the environment, and propose ways to lessen their impact; investigate physical and chemical properties of elements and compounds, and use various methods to visually represent them; demonstrate an understanding of periodic trends in the periodic table and how elements combine to form chemical bonds. Chemical Reactions analyse chemical reactions used in a variety of applications, and assess their impact on society and the environment; investigate different types of chemical reactions; demonstrate an understanding of the different types of chemical reactions. Quantities in Chemical Reactions analyse processes in the home, the workplace, and the environmental sector that use chemical quantities and calculations, and assess the importance of quantitative accuracy in industrial chemical processes; investigate quantitative relationships in chemical reactions, and solve related problems; demonstrate an understanding of the mole concept and its significance to the quantitative analysis of chemical reactions. Solutions and Solubility analyse the origins and effects of water pollution, and a variety of economic, social, and environmental issues related to drinking water; investigate qualitative and quantitative properties of solutions, and solve related problems; demonstrate an understanding of qualitative and quantitative properties of solutions. Gases and Atmospheric Chemistry analyse the cumulative effects of human activities and technologies on air quality, and describe some Canadian initiatives to reduce air pollution, including ways to reduce their own carbon footprint; investigate gas laws that explain the behaviour of gases, and solve related problems; demonstrate an understanding of the laws that explain the behaviour of gases. Scientific Investigation Skills and Career Exploration demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analysing and interpreting, and communicating); identify and describe careers related to the fields of science under study, and describe the contributions of scientists, including Canadians, to those fields. Learning Strategies It is important that students have opportunities to learn in a variety of ways: individually and cooperatively; independently and with teacher direction; through hands-on activities; and through the study of examples followed by practice. The expectations in science courses call for an active, experimental approach to learning, and require all students to participate regularly in laboratory activities. Laboratory activities can reinforce the learning of scientific concepts and promote the development of the skills of scientific investigation and communication. Where opportunity allows, students might be required, as part of their laboratory activities, to design and conduct research on a real scientific problem for which the results are unknown. Evaluation Students will be evaluated on both the theoretical and the practical material presented in this course. A major test will conclude each unit. Assignments will be given frequently. Laboratory investigations will be assessed/evaluated based on submitted lab reports as well as the practical skills preformed during the investigation. Students will also be required to complete unit projects during the semester. Students will be evaluated in the four major categories: Knowledge & Understanding, Inquiry, Communication, and Making Connections. Course Work 70% (30% tests and quizzes, 30% lab assignments, 10% other) Final Exam 30% Learning Skills (Independent Work, Teamwork, Organization, Initiative, and Work Habits/Homework) will be reported on the Provincial Report Card. Homework: “Genius is one percent inspiration and ninety-nine percent perspiration.” ~ Thomas A. Edison It is very important that students do their homework and review the concepts! Much of chemistry builds on itself, so if you are not prepared for the day’s lesson with your homework done, you may find yourself falling behind at an exponential rate! While I encourage you to work together on homework assignments (unless stated otherwise), be sure that you understand what you are doing. Absence from class/ missed tests or assignments: Students are expected to catch up on any missed information, labs and assignments. Students who miss performing a lab will have to come in at lunch to complete the lab as practicing these lab skills is an important part of learning chemistry. Should a student be absent for a test with an acceptable reason, the student will write the test the first day back to school. Should a student be absent on a due date for any class assignment or project with an acceptable reason, the student may hand in the assignment the first day back to school. However, if it is a group presentation, the student will receive zero for that portion of the assignment. Extra Help: I will be available for extra help if you need it after school or some lunch hours at pre-arranged times. I’m really looking forward to our semester together!!