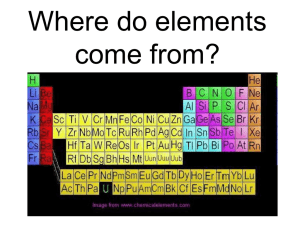
Global Change 1: The Evolution of the Universe
advertisement

Evolution of Stars, Origin of the Elements "In the beginning, there was nothing at all. Earth was not found, nor Heaven above, a Yawning-Gap there was, but grass nowhere." The Edda -- collection of Norse Myths dating to 1200 In this lecture period we learn: Evolution of stars and the H-R diagram (red giant, supernova, black hole) Origin of elements (fusion and neutron capture) Abundances of elements Format for printing Evolution of Stars The scientific evidence is now overwhelming that the Universe began with a "Big Bang." What came next? As the early universe expanded and cooled, matter and anti-matter collided and "annihilated" in a massive process, producing radiation. We believe that there was a slight excess of matter and that hydrogen and helium atoms were "left over." Vast clouds of gas spewing into space led to the formation of galaxies and stars, as matter in local high-density regions collapsed and coalesced under the influence of gravity. Stars were, and continue to be, formed in gaseous nebulae. An example is the Great Orion Nebula, which is one of the youngest features in the sky - thought to be only 20,000 years old. Our own Sun formed in just such a nebula. The Universe is still evolving New stars are being born in gaseous nebulae all the time. Stars have life cycles of over billions of years. The details of the life cycle depend on size and luminosity (brightness). Astronomers have devised ways of studying stellar evolution and since this plays the key role in the origin of elements, we discuss it next. As we saw earlier, the Sun acts as a black body with a temperature of ~6000 K. Other stars seen in the sky are hotter and cooler. Hotter stars are bluer and cooler stars are redder. If you look carefully on a clear and dark night, you can sometimes see these color differences with the eye. Measurements show that stars are in the general range of 3000K to over 20,000 K. These measurements represent the star surface temperatures, not the temperatures inside, which are much hotter and lead to fusion reactions which power the star. Stellar temperatures and luminosity Another characteristic feature that astronomers can measure is the brightness of a star - this depends on both how far away the star is and on characteristics of the star itself. For nearby stars, we can determine distance by geometry, so the brightness can be turned into a real measurement of how much radiation the star emits - its luminosity. For further stars, less direct methods are used. The most important classification scheme for stars plots surface temperature against luminosity. This plot is called the HertzprungRussell (H-R) diagram, illustrated below. Hertzprung-Russell (HR) diagram is a temperature vs. luminosity plot of stars. Some prominent stars are shown by name. The visible colors of stars are indicated at the top, and the different classes of stars are labeled in the diagram. The majority of stars are found to lie on the Main Sequence. Giants and supergiants do not behave as typical stars. The radius of a star can be deduced by its position on the H-R diagram. Can you figure out how, using the Stefan-Boltzmann equation? The Stefan-Boltzmann law relates luminosity and surface area to temperature. Stars to the top right of the diagram are large and stars to the bottom left are small. Mass also changes as a function of position on the diagram. The masses of "Main Sequence" stars range from one-tenth of the Sun's mass at the lowest part, to some 50 or 100 solar masses at the upper end. Heavier stars burn up their fuel more quickly than the smaller stars. Happily for us, the Sun has been on the main sequence for around 4.5 billion years and will remain there for another 4-5 billion years or so. Main Sequence Stars Stellar evolution can be studied using the H-R diagram. The majority of stars spend most of their time on the main sequence burning hydrogen to make helium through fusion reactions in the core. When the hydrogen is used up, the star will move away from the main sequence. A moderately sized star like the sun will become a red giant, growing in size to engulf the Earth and burning helium to make carbon. Following this stage, the outer layers will be thrown off, and the Sun will end up as a white dwarf, a dim star with a very small radius and high density - it will eventually cool and fade from sight. Solar system abundances of the elements. Note that the 1:4 ratio of helium to hydrogen (by weight) is what the Big Bang theory would predict. But other, heaver elements must come from other processes. More importantly for our discussion of the origin of elements is what happens to the massive stars. Before discussing these "supergiants", we need to quickly review the chemical elements. The Figure shows the measured abundances of the elements in our solar system. The major elements in the solar system are hydrogen and helium in exactly the ratio predicted by the Big Bang theory. We need, however, to explain the existence of the heavier elements that could not have been synthesized during the Big Bang itself. Atoms -- a primer The table below list basic characteristics of the building blocks of atoms. Particle Molar Mass Electrical Charge C g/mol electron -1.60217733(49) x E-19 30 0.0005486 Rest Mass kg 0.91093897(54) x E- proton +1.60217733(49) x E-19 1672.6231(10) x E30 1.0072697 neutron 0.0 1674.9543(86) x E30 1.0086650 The atomic number, which is the number of protons, characterizes an atom. In the case of hydrogen, we have one positively-charged proton (the nucleus) that is orbited by one negatively-charged electron. In the other elements, the nucleus of an atom contains both positively-charged protons and neutrally-charged neutrons. The mass number of an atom is the combined weight of protons and neutrons. For our purposes it turns out that electrons have negligible mass compared to the nucleus. Some atoms can have different numbers of neutrons, called isotopes, but if we change the number of protons we change atomic species. The atomic number of H is 1 and its mass is ~1.67E-24 gram; atomic weight is typically expressed as reference mass, and is slightly more than 1 for H (it is 1.0079) because of the presence of other H isotopes. The next element in the periodic system, He, has atomic number 2 (two protons and 2 neutrons) and a mass of ~6.65E-24 gram (or atomic weight of about 4). To make one He atom, we therefore need four H atoms and some modifications. Assuming we are able to overcome the repelling force of the protons, the combined mass of 4 H atoms equals 6.696E-24gram, which exceeds the mass of He. The excess mass is released as energy following Albert Einstein’s well-know E=mc^2 equation; with m is (excess) mass and c is the speed of light. Small as the mass excess may seem, the energy release of only a very small amount of H that fuses to create He is tremendous. This H fusion process is the source of our Sun’s energy, and has also been used to create the most powerful weapon of destruction: the hydrogen bomb. The consequence of the fusion process is a balance between gravitational contraction and expansion due to heat in the Sun. As long as H fusion continues heat will be generated, but what if all H has been converted to He? When H fusion stops, the sun cools and gravitational collapse occurs. This in turn creates heat from the restricted motion of atoms. At some point, enough heat may become available to fuse He atoms, for example into the element C. This releases excess mass in the form of energy as well. Then we have a He-fueled star. Why does H fuse before He? The answer lies in the structure of the nucleus, where a greater number of protons requires more energy to overcome the repelling forces. When He fusion occurs in the core of a star, sufficient heat is generated for H fusion to occur in outer parts of the star. The combined energy these two fusion processes generate causes the star to expand enormously, at which stage we call it a Red Giant. When our Sun reaches this stage 9n 4-5 billion years, its size will exceed the orbit of Earth. Origin of "Heavy" Elements So where do these heavier elements come from? All stars are continually in a balance between gravitational collapse and outward pressure forces due to the fusion reactions in the core. If gravity starts to win, the star collapses and the release of gravitational potential energy allows the star's core to heat up, releasing more energy. Shell structure of a heavy star (~25 solar masses) at the end of its evolution, just prior to a supernova explosion. The fraction of the total mass contained in each shell and the principal elements present are shown. Heavier stars evolve into supergiants, and it is the nuclear reactions in the interiors of these stars that gave rise to the heavy elements in the universe. Such massive stars start by burning hydrogen into helium in the core, then helium to carbon, and then carbon to heavier elements, all the way up to iron. At each stage, once the fuel is consumed in the core, the star contracts and gravitational energy is released, heating the core to temperatures high enough to enable the next stage to begin. The abundant elements carbon and oxygen are made by helium fusion, and the elements up to iron are made in subsequent steps. However, beyond iron most elements are made by successive "neutral capture", though some of the rarer nuclides are made by proton capture reactions. The high temperatures (nearly 1,000,000,000 K) required for this process can only be reached in stars heavier than about 4 solar masses. When the core is composed of heavy elements near iron, no further nuclear fusion is possible and the tendency for the star to collapse under the tremendous gravitational forces is unchecked. As the star's heavy core implodes, huge shock waves break the star apart, spewing the heavier elements out to space in a supernova. Supernovae are fairly common events in distant galaxies, but are only seen rarely in our own, since those within the Milky Way are often obscured by gas and dust. A recently observed supernova was seen in the neighboring Large Magellenic Cloud galaxy (a diffuse "satellite" galaxy of the Milky Way) early in 1987 (on left). Perhaps the most famous supernova was seen by Chinese astronomers in July 1054 and was visible for several weeks in broad daylight. The visible remnant of this huge explosion is now called the Crab Nebula. For a detailed view of the Crab Nebula, look at the recent Hubble Telescope image. This supernova remnant is 6,500 light years away. Another beautiful example of a supernova remnant is the Cygnus Loop, lying about 2,500 light years away (on right). The evolution of even more massive stars produce other objects in our universe. Perhaps the most intriguing object that can form from a massive collapsing star is a black hole. These object are invisible because all matter is unable to escape the gravitational attraction. We use indirect means to recognize their presence and many galaxies, including our own Milky Way galaxy, may have a black hole in their core. Calculations show that only one ninth of the material in the solar system was generated in supernovae, while the remaining eightninths is hydrogen and helium. It is interesting to note that the elemental abundances of our planet is notably different in H and He (below), which we use later to understand the origin of Earth and her immediate neighbors Abundances of the elements in Earth. Comparison with the figure above shows that Earth has lost most of its primordial hydrogen and helium. Summary The elements hydrogen and helium were synthesized in the Big Bang. Higher elements were synthesized in fusion reactions (up to Fe) and neutron capture reactions within massive stars. These elements were dispersed in supernovae. About 10% of the material in the solar system (including the most critical materials making up our bodies) came from supernovae, the rest from the Big Bang. All of us come from the stars .... Suggested Readings Cox, P. A., The Elements: Their Origin, Abundance, and Distribution Oxford Scientific Publications, 1989. Self Test Take the Self-Test for this lecture. All materials © the Regents of the University of Michigan unless noted otherwise.