Determination of the heat of formation of magnesium carbonate
advertisement

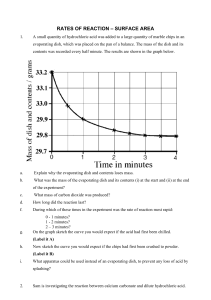
F.6/7 Chemistry Practical: Enthalpy of Formation of Calcium Carbonate Objective: To determine enthalpy of formation of calcium carbonate --------------------------------------------------------------------------------------------------------------------Introduction Molar enthalpy of formation of calcium carbonate is the enthalpy change for the formation of 1 mole of calcium carbonate according to the following equation at s.t.p: In this experiment, enthalpy of formation of calcium carbonate is determined indirectly by measuring the following molar enthalpy change (1) Ca(s) + 2HCl(aq) -------------> CaCl2(aq) + H2(g) H 1 (2) CaCO3(s) + 2HCl(aq) --------> CaCl2(aq) + CO2(g) + H2O(l) H 2 ----------------------------------------------------------------------------------------------------------------------Theory (Write this section with the help of the following questions) 1. Work out an energy cycle to relate Hf, H 1 and H 2 . [Hints: molar enthalpy of formation of water, -285 kJ mol-1 and molar enthalpy of formation of carbon dioxide, -393 kJ mol-1 are necessary data in the energy cycle.] 2. In procedure A, temperature change, , for the reaction of calcium and hydrochloric acid is determined. Give a formula for the calculation of 'heat given out, Q' by using the temperature change. (Use the following symbols in your formula: mw for mass of hydrochloric acid, Cw for specific heat capacity of the resulting solution, for the temperature change) 3. Show that calcium metal is the limiting reagent in step 3 of procedure A. 4. If x g of calcium is used to react with 150 cm3 of hydrochloric acid, express H1 in terms of Q and x. -------------------------------------------------------------------------------------------------------------------Chemicals: 0.9 g calcium, 2.5 g anhydrous calcium carbonate (in powder form), 300 cm3 of 2 M hydrochloric acid Apparatus: -10 - 110 oC thermometer, readable 0.5 oC, polystyrene cup with cover, capacity of 200 cm3 and electronic balance, readable 2 digits. Procedure A. Reaction of calcium with dilute hydrochloric acid 1. Weigh out approximate 0.9 g of calcium, recording the mass to two decimal places. 2. Using a measuring cylinder, measure out 150 cm3 of 2 M hydrochloric acid and place it in a polystyrene beaker. Measure the temperature of the acid. 3. Add the weighed portion of calcium metal into the acid and stir thoroughly with the thermometer until all the metal has reacted. 4. Record the maximum temperature attained by the solution. (If there is time, repeat the experiment and determine the average temperature rise per gram of calcium used.). B. Reaction of calcium carbonate with dilute hydrochloric acid 1. Weigh out 2.5 g of dry, powdered calcium carbonate on a piece of clean paper. Transfer the En03_CaCO3 /p.1 carbonate into a clean polystyrene beaker. 2. 3. 4. Record the mass to one decimal places. Place 150 cm3 of approximate 2 M hydrochloric acid in a measuring cylinder. Record its temperature Pour the acid on the carbonate in the beaker. Stir briskly with the thermometer and record the maximum temperature reached by the solution. (If there is time, repeat the temperature and determine the average temperature rise per gram of magnesium carbonate used.) Data and results [You should prepare the table of data before going to the laboratory for practical] A suggested format of data table is given below. Procedure A Procedure B Mass of Ca + container /g Mass of CaCO3+container /g Mass of container /g Mass of container /g Mass of Ca used /g Mass of CaCO3 used /g o Final temperature / C Final temperature /oC Initial temperature /oC Initial temperature /oC Temperature rise /oC Temperature rise /oC Calculation Calculate molar enthalpy of formation of calcium carbonate. Discussion (Write this section with the help of the following questions) 1. Which reagent is in excess in step 1-2? Calcium or hydrochloric acid? Show that one of them is in excess by calculation. 2. It is desirable that one of the reagents is in excess. Explain. 3. What assumptions have you made in the calculation? 4. Give (a) random error sources and systematic error sources in this practical. 5. Try to estimate percentage error of H 1 and H 2 . 6. On which thermodynamic principle does Hess's law of constant heat summation depend? 7. Can you measure molar enthalpy of formation directly from the reaction between carbon, oxygen and calcium? Why? ________________________________END__________________________________ En03_CaCO3 /p.2