Coulombic Attraction and Periodicity Definitions
advertisement
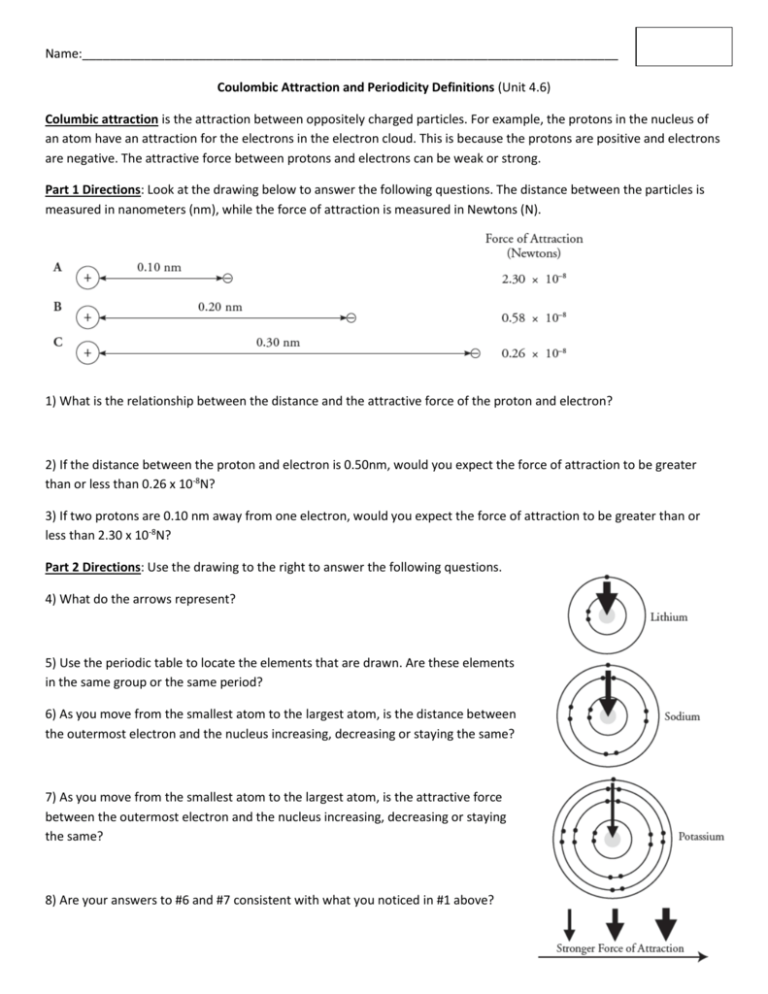
Name:______________________________________________________________________________ Coulombic Attraction and Periodicity Definitions (Unit 4.6) Columbic attraction is the attraction between oppositely charged particles. For example, the protons in the nucleus of an atom have an attraction for the electrons in the electron cloud. This is because the protons are positive and electrons are negative. The attractive force between protons and electrons can be weak or strong. Part 1 Directions: Look at the drawing below to answer the following questions. The distance between the particles is measured in nanometers (nm), while the force of attraction is measured in Newtons (N). 1) What is the relationship between the distance and the attractive force of the proton and electron? 2) If the distance between the proton and electron is 0.50nm, would you expect the force of attraction to be greater than or less than 0.26 x 10-8N? 3) If two protons are 0.10 nm away from one electron, would you expect the force of attraction to be greater than or less than 2.30 x 10-8N? Part 2 Directions: Use the drawing to the right to answer the following questions. 4) What do the arrows represent? 5) Use the periodic table to locate the elements that are drawn. Are these elements in the same group or the same period? 6) As you move from the smallest atom to the largest atom, is the distance between the outermost electron and the nucleus increasing, decreasing or staying the same? 7) As you move from the smallest atom to the largest atom, is the attractive force between the outermost electron and the nucleus increasing, decreasing or staying the same? 8) Are your answers to #6 and #7 consistent with what you noticed in #1 above? Name:______________________________________________________________________________ Part 3 Directions: Look at the drawing below to answer the following questions. The distance between the particles is measured in nanometers (nm), while the force of attraction is measured in Newtons (N). 9) What is the relationship between the number of protons and the attractive force? 10) What would be the attractive force on a single electron if there were five protons in the nucleus of an atom? Show your work. 11) Imagine that a second electron were placed to the left of a nucleus containing two protons (scenario D). What would be the attractive force on the original electron and the second electron? Part 4 Directions: Look at the drawing below to answer the following questions. 12) Using the periodic table, locate the elements in the drawing above. Correctly identify and label the number of protons for each atom above. Are the elements in the same group or period? 13) Which of the three atoms in the drawing above has the strongest attraction for its outermost electron(s)? 14) As you move from left to right does the distance between the nucleus and the outermost electron(s) change significantly? Do you think the distance has an impact on the attractive force between the outermost electrons and the nucleus in the drawing above? 15) Is there a correlation between the number of protons and the attractive force between the outermost electron(s) and the nucleus? If so, what is the relationship? Name:______________________________________________________________________________ Part 5 Directions: Label the number of protons on each atom in the drawing in Part 2. Refer back to that drawing to answer the following question. 16) When comparing the elements in the same column of the periodic table, which factor (distance to the nucleus or number of protons in the nucleus) seems to be the dominant factor for determining the attractive force between the outermost electron(s) and the nucleus? Explain. Columbic Attraction Summary 1) As the distance between the nucleus and an electron increases the attractive force _________________________. 2 If the amount of protons in the nucleus increases, than the attraction between the nucleus and the electron will __________________________. 3) Across a period, the distance between the outermost electron(s) and the nucleus ____________________, while the number of protons in the nucleus ___________________. This causes the attractive force between the nucleus and the outermost electron(s) to ___________________. 4) Down a group, the distance between the outermost electron(s) and the nucleus _____________________, while the number of protons in the nucleus _________________. This causes the attractive force between the nucleus and the outermost electron(s) to _____________________. 5) For each set of elements below, circle the element whose atoms will have a stronger attractive force between their outermost electron(s) and the nucleus. Ca or Ba Cr or Cu Ar or Xe B or F Target 4.6 Definitions Directions: Read the definitions of the terms below. Be ready to identify them on a short quiz tomorrow. Atomic radius- The distance from the nucleus to the outermost electron. Ionization Energy- The amount of energy needed to remove an electron from an atom. Electronegativity- The ability of an atom’s nucleus to attract an additional electron from a different atom.









