Ideal Gas Law in Meteorology
advertisement

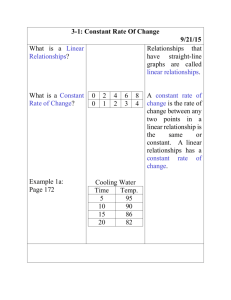
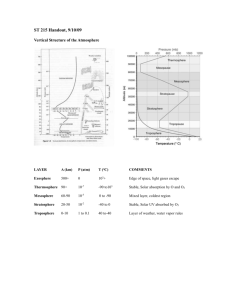
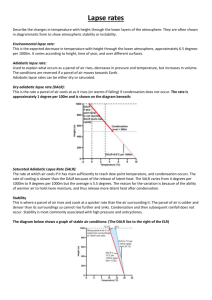
Ideal Gas Law in Meteorology - Simple equation that relates pressure, temperature, and density or volume Very relevant to meteorology Density: mass of air per unit volume. Decreases as one moves up in atmosphere. Pressure: weight of air above you. Decreases as one moves up in atmosphere. - - - - Equation given by pV = nRT o Where p = pressure, V = volume, n = # of moles of gas, R = gas constant, and T = temperature Other ways to write it o pV = mRdT, where m = mass, Rd = gas constant for dry air o Also p = mRdT = p = ρRdT o V o density of gas () = mass/volume For our purposes, since the constants are exactly that—we can ignore them and just look at the relationship between T, p, and ρ or T, p, and V Giving: o p X V is proportional to T any change in temperature must be balanced out by the product of pressure and volume If temperature is constant, p and V are inversely proportional (if one goes up, the other must go down) o p is proportional to X T any change in pressure must be balanced out by the product of temperature and density So… o Adiabatic compression states that as air sinks, it warms due to compression. o Adiabatic expansion states that as air rises, it cools and expands. Who cares? - Explains why o Marshmallows expand in the microwave o Bags of chips/shampoo bottles expand when you fly or drive up to the mountains o Cold objects tend to shrivel up o Etc. Questions to think about: Illustrate the answers using the gas law 1) If the temperature of air rises, but the volume stays the same, what happens to the pressure? 2) If the temperature of an object falls, but the density stays the same, what happens to the pressure? 3) If the temperature of an inflated balloon is cooled, but remains at the surface, will the balloon expand or shrink? 4) As a parcel of air (a uniform volume of air) rises up in the atmosphere, what will happen? What happens when the same parcel sinks?