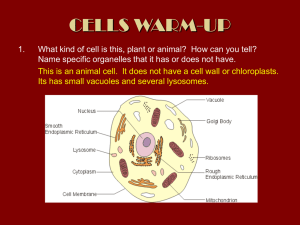
Chloroplast Isolation

1.
http://homepages.gac.edu/~cellab/chpts/chpt8/ex8-1.html
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Isolation of Chloroplasts from Spinach Leaves
Materials
*
*
Fresh spinach leaves
Grinding solution
0.33 M Sorbitol
10 mM Sodium pyrophosphate (NaPO)
4 mM MgCl
2 mM Ascorbic Acid
Adjust pH to 6.5 with HCl
Chopping board and knife
Chilled mortar and pestle
Cheesecloth
Refrigerated preparative centrifuge
Suspension solution
0.33 M Sorbitol
2 mM EDTA
1 mM MgCl
50 mM HEPES
Adjust pH to 7.6 with NaOH
Hemacytometer and microscope
Procedure
1.
2.
Prepare an ice bath and pre-cool all glassware to be used.
Select several fresh spinach leaves and remove the large veins by tearing them loose from the leaves. Weigh out 4.0 grams of deveined leaf tissue.
3. Chop the tissue as fine as possible. Add the tissue to an ice-cold mortar containing
15 ml of grinding solution and grind to a fine paste.
4. Filter the solution through double layered cheesecloth into a beaker and squeeze the tissue pulp to recover all of the suspension.
5. Transfer the green suspension to a cold 50 ml. centrifuge tube and centrifuge at
200 xg for 1 minute at 4° C to pellet the unbroken cells and fragments.
6. Decant the supernatant into a clean centrifuge tube and recentrifuge at 1000 xg for
7 minutes. The pellet formed during this centrifugation contains chloroplasts. Decant and discard the supernatant.
7. Resuspend the chloroplast pellet in 5.0 ml. of cold suspension solution or 0.035 M
NaCl. Use a glass stirring rod to gently disrupt the packed pellet. This is the chloroplast suspension for use in subsequent procedures.
8. Enclose the tube in aluminum foil and place it in an ice bucket. 1
9. Determine the number of chloroplasts/ml of suspension media using a hemocytometer.
Record the # of chloroplasts/ml_________
Notes
The isolation procedure used here leaves the chloroplast outer membrane intact.If you wish to study the enzymes for photophosphorylation, wash the chloroplasts and rupture the outer membranes. To rupture the outer membranes, resuspend the chloroplasts in diluted suspension solution (1:25). Immediately centrifuge the chloroplast suspension at
8,000 xg for 5 minutes to collect the chloroplasts. Remove the diluted suspension media and resuspend the chloroplasts in isotonic media (0.35 M NaCl or undiluted suspension buffer).
------------------------------------------------------------------------
Cell Biology Laboratory Manual
------------------------------------------------------------------------
Dr. William H. Heidcamp, Biology Department, Gustavus Adolphus College,
St. Peter, MN 56082 -- cellab@gac.edu
2.
Plant Physiology
58:309-314 (1976)
Isolation of Intact Chloroplasts and Other Cell Organelles from
Spinach Leaf Protoplasts
Mikio Nishimura, Douglas Graham2 and Takashi Akazawa
Research Institute for Biochemical Regulation, School of Agriculture, Nagoya
University, Chikusa, Nagoya 464, Japan
Freshly prepared spinach leaf protoplasts were gently ruptured by mechanical shearing followed by sucrose density gradient centrifugation to separate constituent cell organelles. The isolation of intact Class I chloroplasts ( d = 1.21) in high yield, well separated from peroxisomes and mitochondria, was evidenced by the specific localization of ribulose-1,5-bisphosphate carboxylase (EC 4.1.1.39), NADP triose-P dehydrogenase
(EC 1.2.1.9), and carbonic anhydrase (EC 4.2.1.1) in the fractions. A clear separation of chloroplastic ribosomes from the soluble cytoplasmic ribosomes was also demonstrated by the band patterns of constituent RNA species in the polyacrylamide gel electrophoresis. Localization of several enzyme activities specific to leaf peroxisomes, e.g.
catalase (EC 1.11.1.6), glycolate oxidase (EC 1.1.3.1), glyoxylate reductase (EC
1.1.1.26), glutamate glyoxylate aminotransferase (EC 2.6.1.4), serine glyoxylate aminotransferase, and alanine glyoxylate aminotransferase (EC 2.6.1.12) in the peroxisomal fractions ( d = 1.25), was demonstrated. Overall results show the feasibility of the method for the isolation of pure organelle components in leaf tissues.
3.
http://www.sigmaaldrich.com/Area_of_Interest/Life_Science
/Plant_Biotechnology/Chloroplast_Iso_Kit.html
Chloroplast Isolation Kit
Plant Proteomics and Protein Expression
Description:
The Chloroplast Isolation Kit provides a quick and efficient procedure to isolate intact chloroplasts from plant leaves. Intact chloroplasts are the best starting material for studies of chloroplastic processes such as carbon assimilation, electron flow and phosphorylation, metabolic transport, or protein targeting. The chloroplast fraction can be further extracted to obtain membrane, stroma, or thylakoid proteins as well as chloroplastic DNA and RNA.
The chloroplast isolation method includes mechanical cell wall and membrane breakage, removal of cell debris and unbroken leaf tissue by filtration, collection of total cell chloroplasts by centrifugation, and separation of intact from broken chloroplasts usi ng a Percoll® layer or gradient. The Chloroplast Isolation Kit has been tested for use with spinach, pea, lettuce, cabbage, mangold, and tobacco.
Kit Components:
*
*
*
Chloroplast Isolation Buffer 5x (CIB)
Percoll
Bovine Serum Albumin (BSA)
Filter Mesh 100 *
------------------------------------------------------------------------
Ferricyanide photoreduction - a measure of intact chloroplasts
This assay is based upon the inability of the ferricyanide to cross the chloroplast envelope and to react with the electron transport system in the thylakoid membranes. Ferricyanide reduction, as indicated by the decrease in the absorbancy at 410 nm, occurs only when ruptured chloroplasts are present in the preparation.
The degree of integrity of the chloroplast preparation is assessed by comparing the rate of ferricyanide reduction upon illumination before and after osmotic shock of the chloroplasts.
Analysis of the results, presented here, indicates that 88% of the chloroplasts in the spinach chloroplast preparation are intact.
Chloroplasts (equivalent to 25 µg/ml chlorophyll) prepared using CP-ISO were illuminated in the presence of 1.5 mM ferricyanide. The reduction of ferricyanide was measured spectrophotometrically (410 nm).
Graph A demonstrates the change in absorbancy of the two samples (before and after osmotic shock) during 6 minutes.
Graph B
shows bars representing the slopes of the lines in Graph A.
Ordering Information
Product Product Name Package Size
CP-ISO Chloroplast Isolation Kit
Site Use Terms |
Privacy |
1 Kit X
Technical Bulletin
Terms and Conditions of Sale |
Business Development | Contact
Us
Copyrights © 2008 Sigma-Aldrich Co. All Rights Reserved.
Reproduction of any materials from the site is strictly forbidden without permission.
Sigma-Aldrich brand products are sold exclusively through Sigma-
Aldrich, Inc.
4. http://www.springerprotocols.com/Abstract/doi/10.1385/0-89603-236-
1:123
Isolation and Purification of Functionally Intact Chloroplasts from Leaf Tissue and
Leaf Tissue Protoplasts
By: David G. Whitehouse , Anthony L. Moore
Abstract
The isolation of photosynthetically active chloroplasts is the starting point for many plant metabolic studies as diverse as carbon assimilation, electron flow and phosphorylation, metabolite transport, and protein targeting. Whatever the subsequent use of the chloroplasts, it is preferable to attempt to isolate intact chloroplasts, as the organelle envelope will protect the stroma and thylakoids from the deleterious effects of degradative enzymes and phenolic compounds released from leaf tissues during the preparative procedures.
Affiliation(s): (3) Department of Biochemistry, University of Sussex, Brighton, UK
Book Title: Biomembrane Protocols: I. Isolation and Analysis
Series: Methods in Molecular Biology | Volume: 19 | Pub. Date: Aug-20-
1993 | Page Range: 123-131 | DOI: 10.1385/0-89603-236-1:123