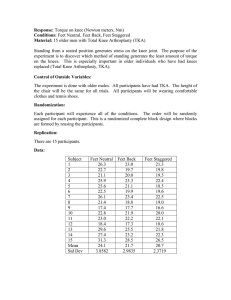
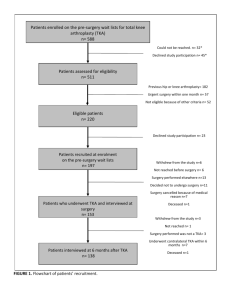
Continuous Passive Motion Devices
advertisement

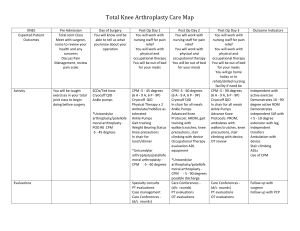
REVIEW REQUEST FOR Continuous Passive Motion Devices Provider Data Collection Tool Based on Medical Policies 1.01.10 DME.00019 Policy Last Review Date: 07/2010; 08/19/2010 Policy Effective Date: 07/2010; 10/13/2010 Provider Tool Effective Date: 02/15/2011 Individual’s Name: Date of Birth: Insurance Identification Number: Individual’s Phone Number: Ordering Provider Name & Specialty: Provider ID Number: Office Address: Office Phone Number: Office Fax Number: Rendering Provider Name & Specialty: Provider ID Number: Office Address: Office Phone Number: Office Fax Number: Facility Name: Facility ID Number: Facility Address: Date/Date Range of Service: Service Requested (CPT if known): Place of Service: Outpatient Home Inpatient Other: Diagnosis (ICD-9) if known): Please check all that apply to the individual: Request is for Continuous Passive Motion (CPM) device applied within 72 hours following surgery Request is for CPM use for longer than 21 days from date of first application Other: (please list) Individual is status post anterior cruciate ligament (ACL) repair Individual is status post articular osteochondral repair procedures of the knee (such as microfracture, osteochondral grafting, autologous chondrocyte implantation (ACI), meniscal allograft, treatment of osteochondritis dissecans, repair of tibial plateau fractures Individual is status post posterior cruciate ligament (PCL) repair Individual is status post total knee arthroplasty (TKA) or TKA revision Request is subsequent to arthroplasty or the release of an arthrofibrosis of the elbow or knee, shoulder, wrist or hand. (e.g. elbow arthroplasty, rotator cuff repair or arthroplasty or metacarpal joint phalangeal arthroplasty) Request is for any of the following (check all that apply): Treatment of ankle or toe conditions Treatment of chronic contractures Treatment of degenerative joint diseases Treatment of distal radial fractures Treatment of idiopathic club foot in infants Treatment of stroke with hemiparesis Treatment of TMJ (temporomandibular joint) Other (explain): Device was initially applied in an inpatient setting prior discharge Date of surgery Date of device application Discharge date Other uses: (please list) Codes: Procedure Code(s): ________________________ Diagnosis Code(s): ________________________ This request is being submitted: Pre-Claim Post–Claim. If checked, please attach the claim or indicate the claim number I attest the information provided is true and accurate to the best of my knowledge. I understand that Anthem may perform a routine audit and request the medical documentation to verify the accuracy of the information reported on this form. _____________________________________________________________ Name and Title of Provider or Provider Representative Completing Form and Attestation (Please Print)* Date *The attestation fields must be completed by a provider or provider representative in order for the tool to be accepted Page 2 of 2