ch 1 question
advertisement

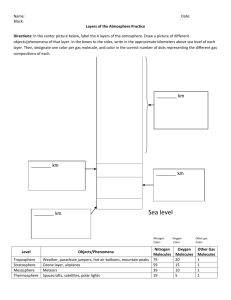
Name: ____________________________Date: _____________________Pd. ___________ THE ATMOSPHERE: CHAPTER 1 Directions: Define the key vocabulary terms from Chapter 1. Answer all assessment questions in complete sentences. Chapter 1.1: The Air Around You I. ANSWERS Vocabulary: a. Weather – the condition of the Earth’s atmosphere at a particular time and place b. Atmosphere: the envelope of gases that surrounds the planet c. Ozone: a form of oxygen with three atoms in each molecule. d. Water vapor: water in the form of a gas II. Assessment Questions 1. What are the four most common gases in dry air? Nitrogen, oxygen, argon, carbon dioxide 2. What are three ways in which the atmosphere is important to life on Earth? Contains oxygen needed by MANY organisms, provides warmth, protects from dangerous UV radiation and objects from space. 3. How would the amount of carbon dioxide in the atmosphere change if there were no plants? Carbon dioxide levels would increase if there were no plant life to use it for photosynthesis 4. How would Earth be different without the atmosphere? More than likely, no life could exist. Chapter 1.2: Air Pressure I. Vocabulary: a. Density: the amount of mass in a given volume. D = M/V b. Pressure: the force pushing on an area or surface. 1 c. Air pressure: the result of the weight of a column of air pushing down in an area. d. Barometer: an instrument used to measure air pressure e. Mercury barometer: a barometer that used mercury, as air pressure pushes down on the surface of the mercury, the mercury will rise up the tube. f. Aneroid barometer: a barometer “without liquid”, an airtight metal chamber responds by changing shape due to the air pressure, changes are recorded by a series of springs and levers g. Altitude: The distance above sea level. II. Assessment Questions 1How does increasing the density of a gas affect its pressure? The pressure increases 2. What units are commonly used to measure air pressure? Millibars 3. How many milibars are equal to 27.23 inches of mercury? 1 inch of mercury = 33.87 milibars. Therefore, 27.23 inches of mercury X 33.87 = 922.3 millibars 4. As altitude increases, how does air pressure change? Air pressure decreases. How does density change? Density decreases 5. What changes in air pressure would you expect if you carried a barometer down a mine shaft? Air pressure would increase – the amount of air above you would increase thus increasing the “weight” or pressure of the air above you. Chapter 1.3: Layers of the Atmosphere I. Vocabulary: a. Troposphere: the lowest layer of Earth’s atmosphere ( closest to the planet surface) b. Stratosphere: extends from the top of the Troposphere about 50 Km up. c. Mesosphere: the middle layer of the atmosphere (50km to 80km up) d. Thermosphere: the outer most layer of the Earth’s atmosphere. (80km and beyond) e. Ionosphere: sub-layer of the troposphere, contains electrically charged particles 2 f. II. Exosphere: outer portion of the thermosphere, 400Km to beyond…..) Assessment Questions: 1. What properties are used to distinguish the layers of the atmosphere? Temperature and objects commonly found within the layer and any unique characteristics. 2. According to Figure 9, in which layer of the atmosphere do communications satellites orbit? Exosphere or Thermosphere 3. Give at least one important characteristic of each of the four main layers of Earth’s atmosphere. T = weather occurs; S = ozone layer; M = meteoroids burn up; TH = temps extremely high 4. How does temperature change as height increases in the troposphere? Compare this to how temperature changes with height in the stratosphere. Temp of the Troposphere decreases with height, temp of the stratosphere generally increases with height. Chapter 1.4: Air Quality I. Vocabulary a. Pollutants: Harmful substances in the air, water or soil b. Photochemical smog: a smog formed by the action of sunlight on pollutants such as hydrocarbons and nitrogen oxides. These chemicals react to form a brown mixture of ozone and other pollutants. c. Acid rain: rain that is more acidic than normal due to air pollution. Nitrogen oxides and sulfur oxides combine with water in the air to form nitric acid and sulfuric acid. II. Assessment Questions: 1. Name three natural processes and three human activities that cause air pollution. Natural = molds, pollen, and particles from forest fires, volcanic eruptions, dust storms. Human = farming, construction, burning fossil fuels. 2. What human activity is responsible for the formation of smog and acid rain? Burning fossil fuels. 3 3. What kind of harm does photochemical smog cause? It can irritate eyes, lungs, and throat, harm plants, and damage certain materials. 4. Do you think that photochemical smog levels are higher during the winter or during the summer? Explain. In the summer. The sun’s rays are more direct and the hours of daylight are longer. 6. How and why has the air quality changed in the United States over the last 30 years? It has generally improved because of new cars, power plants that pollute less, and laws that have reduced pollution. 4