Kautschuk-Historie
advertisement

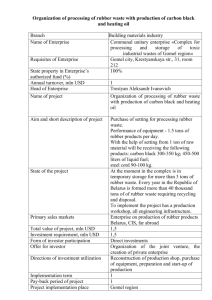
News Release LANXESS at Rubber Days 2006 From tree to street LANXESS Corporation Contact: Terri Fitzpatrick Vice President Communications North America 111 RIDC Park West Drive Pittsburgh, PA 15275-1112 How LANXESS transformed rubber into the flexible, highperformance tire materials we know today New York, NY – August 28, 2006 – What do we actually know about the round black “gumshoes” on our automobiles, which we rely on every day. Probably not many people know that the tires on our cars are made from high-tech materials over which countless research scientists have racked their brains for decades: it was a long journey from the “original” rubber discovered by the Aztecs to the tires of today and the comfort and safety they offer us. The invention of rubber The “black art” as “rubber insiders” refer to their work, began with a sticky compound that 18th century explorers found among the South American natives and later brought to Europe. The secret of the rubber tree, Hevea brasiliensis, initially attracted little attention. However, all that changed when the first amateur scientists found a use for rubber first known as “caoutchouc” from its original name “Cau utchu” or “weeping tree”. This rubber was used as a coating for raincoats for Britain’s damp island climate. There were considerable problems associated with the first rubber products - they became hard as a rock in cold weather and soft and sticky in hot weather. The first person to resolve these problems was an American inventor by the name of Charles Goodyear, who in 1839 succeeded in producing the first elastic rubber from the rudimentary, sticky raw product. Admittedly this all happened rather by chance, since in his exploratory zeal the rubber-obsessed scientist mixed virtually anything that came within reach of his laboratory bench with the object of his passion. It is said that he even tried mixing chicken soup with rubber to see if Phone: 412-809-1516 Fax: 412-809-3603 terri.fitzpatrick@lanxess.com Page 1 of 5 something new might come out of it – although little ever did. When he happened upon sulfur during his experiments and left the mixture next to his stove, he had a breakthrough. This treatment altered the properties of the rubber completely: it remained elastic and lost its stickiness. Goodyear had vulcanized the first rubber and in doing so had laid the foundation for elastic materials with long-term serviceability. This breakthrough was soon followed by the invention of the rubber tire, although it took some time for the new tires to catch on. The first automobiles had just been invented but on the bumpy roads of the day it made little difference whether you clattered towards your destination on wooden or rubber wheels. With the introduction of pneumatic tires, however, things really took off: the tires were so popular that rubber, which was still grown on plantations, became more and more scarce and consequently more expensive. Race to invent super rubber This brought chemists into the arena. In 1906, the management of a well-known Leverkusen chemical company offered a reward of 20,000 German Marks – a tidy sum back in those days – to any research chemist who could succeed in discovering a synthetic substitute for rubber. After three years of intensive work, a Leverkusen chemist by the name of Fritz Hofmann was the first person to synthesize what actually grows on trees: synthetic rubber. This made him the father of modern rubber, and the company he worked for – now LANXESS – one of the biggest rubber manufacturers in the world. This was far from the end of rubber research. In fact, it was just the beginning. Hofmann had not made a 1:1 copy of the natural material; he had only imitated its structure in a test tube with raw materials that he modeled after the natural material. Despite this, the scientist had a fair amount of success. The German Kaiser treated his limousine to tires made from Hofmann’s “hip” new methyl rubber. Unfortunately, his pleasure in the new tires did not last long – as the tires began to crumble away under the influence of sunlight. This simply spurred on the Leverkusen rubber chemists. Over the following decades they experimented with new methods of rubber synthesis and tried out new materials until they finally came up with a LANXESS Corporation Contact: Terri Fitzpatrick Vice President Communications North America 111 RIDC Park West Drive Pittsburgh, PA 15275-1112 Phone: 412-809-1516 Fax: 412-809-3603 terri.fitzpatrick@lanxess.com Page 2 of 5 rubber called “Buna S”, which exceeded all expectations. This styrenebutadiene rubber (SBR), which contained not only building blocks based on the natural material but also other molecular links from reactors to the chemical industry, was significantly more resilient than natural rubber. LANXESS Corporation Contact: Terri Fitzpatrick Vice President Communications North America 111 RIDC Park West Drive Pittsburgh, PA 15275-1112 Forefather of today’s high-performance rubber Buna S opened up the way for modern tires, and is still the most important synthetic rubber. But this was not the end of the story. The chemical reaction that led to Buna S could be varied; using similar formulations the Leverkusen rubber experts developed more and more varieties of rubber with amazing properties – even though they had done very little to the basic rubber formulation developed by rubber pioneers at the turn of the century. Only the extra ingredients had improved. Even today rubber manufacturers are still using these rubbers to produce rubber materials with an enormous variety of properties, all depending on the type of rubber and the filler, plasticizer and additives used; the range extends from chewing gum soft to hard as a board, “hydrophilic” to “black ice resistant”. All of these different types of rubber that can be produced from LANXESS raw materials are absolutely essential today. Reputable tire manufacturers now commonly use over ten different rubber blends for their tires. This is because the particularly air-tight inner wall of the tire, has to be made differently from the tread: the sidewall of the tire in turn has to withstand enormous pressing and squeezing forces as the wheel rotates, while good road holding capability is particularly important for the tread. It is important to remember that for even the fastest sports car the only contact with the ground is via the four palm-sized areas of the tires actually touching the road surface. These comparatively tiny areas not only have to transfer the power of the engine onto the road when the vehicle accelerates but also have to withstand the much greater negative acceleration values when it brakes. Only synthetic rubbers like SBR can do this – without it the speeds at which we are accustomed to traveling today would simply not be possible. Phone: 412-809-1516 Fax: 412-809-3603 terri.fitzpatrick@lanxess.com Page 3 of 5 Road handling is of course a tricky issue in tire chemistry. Soft rubber blends hold the road well but are “abraded” too quickly – as is demonstrated by the extremely soft racecar practice tires, which survive for only a few laps. Hard rubber blends last longer, but do not hold the road well. Tire manufacturers have to find compromises, which can often only be achieved with tailor-made, ultra-tough yet soft specialpurpose rubbers cooked up by the chemical industry. Conflicting aims and how to resolve them Anyone who has ever slipped on a wet step when wearing rubber-soled shoes will understand why tire manufacturers are concerned about improving the wet grip of their products. Rubber blends that could cope with wet surfaces used to have a relatively high rolling resistance, which meant that they required too much gas. Tire experts found out how to resolve this problem when they supplemented the conventional filler, carbon black, with silica. The chemical composition of silica is similar to that of ordinary sand and gives the tire good grip in wet conditions – without affecting rolling resistance. Silica is more difficult to process, however, because it does not mix particularly well with standard rubber. Here again, the experience of LANXESS’s rubber chemists came into play: they developed a special rubber with special silica-compatible “anchor blocks”, which are firmly incorporated into the rubber molecule. This rubber naturally bonds extremely well with silica. Weather conditions at the opposite end of the scale – intensive sunlight, extreme temperature changes and heat – are just as much of a problem. These conditions also make the various materials from the rubber family difficult to deal with, as they gradually destroy the molecules in the rubber. Unprotected tires start to crack at the surface, become brittle, and gradually decompose. This problem too has since been resolved. Chemists from LANXESS learned a lot from history and over the decades they developed a whole range of special additives to protect tires from the damaging influence of UV light. About LANXESS LANXESS Corporation Contact: Terri Fitzpatrick Vice President Communications North America 111 RIDC Park West Drive Pittsburgh, PA 15275-1112 Phone: 412-809-1516 Fax: 412-809-3603 terri.fitzpatrick@lanxess.com Page 4 of 5 The LANXESS-Group manufactures high-quality products in the areas of chemicals, synthetic rubber and plastics. The company's portfolio comprises basic and fine chemicals, color pigments, plastics, synthetic rubber and rubber chemicals, leather, textile processing chemicals, material protection products and water treatment products. LANXESS Corporation was formed when the Bayer Group combined most of its chemical businesses and large segments of its polymer activities. The company began operating as a legal entity in the United States on July 1, 2004. LANXESS Corporation is a member of the German LANXESS-Group that was spun-off from Bayer in January 2005. Information for editors: All our news releases and photos can be found on the LANXESS homepage at www.US.LANXESS.com under the "News and Events" button. Forward-looking statements This news release contains forward-looking statements based on current assumptions and forecasts made by LANXESS AG management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. The company assumes no liability whatsoever to update these forward-looking statements or to conform them to future events or developments. LANXESS Corporation Contact: Terri Fitzpatrick Vice President Communications North America 111 RIDC Park West Drive Pittsburgh, PA 15275-1112 Phone: 412-809-1516 Fax: 412-809-3603 terri.fitzpatrick@lanxess.com Page 5 of 5