Lab
advertisement

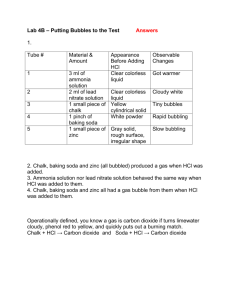
Lab: Less than Zero Submitted by: Steve Sogo Laguna Beach High School in Laguna Beach, California Thanks to: PASCO FOR THE TEACHER Summary In this lab, students will take investigate the endothermic reaction between baking soda and HCl. Students will consider stoichiometric ratios, molar concentrations, reaction scale, and calorimetry. The lab starts with a scripted reaction that uses a given molar ratios, a glass beaker, and 2-M HCl, and they will witness a temperature drop of about 5 to 8 C. Students then adjust the experiment so they can achieve a temperature drop of more than 20 C. Resource Type Lab Grade Level High school Objectives By the end of this lesson, students will Gain an understanding and witness firsthand an endothermic reaction. Use stoichiometry to influence a thermodynamic result. Chemistry Topics This lesson supports students’ understanding of Thermodynamics Stoichiometry Calorimetry Time Teacher Preparation: 30–90 minutes Lesson: 90 minutes (45 minutes to experiment, 45 minutes to discuss variables to change) Materials For each group: Computer with Data Studio installed PASCO temperature probe PASCO USB port Balance 250-mL beaker (3) Baking soda (NaHCO3) 25-mL graduated cylinder 1-M HCl 2-M HCl 3-M HCl 6-M HCl Safety Always wear safety goggles when working in the lab. When working with acids, if any solution gets on students’ skin, they should immediately alert you and thoroughly flush their skin with water. Always add acid to water when diluting acid. Students should wash their hands thoroughly before leaving the lab. When students complete the lab, instruct them how to clean up their materials and dispose of any chemicals. Teacher Notes This lab is an example of real-world problem solving that is highly motivating for students. My students typically have vociferous debates about how best to proceed. The experimental results provide immediate feedback on whether the ideas pursued by the group have validity. Steve Sogo’s tutorial of how to use PASCO probe to collect temperature data: http://youtu.be/pDgQbjMaq2k Money-Saving Tip: Fairly concentrated solutions of HCl can be purchased at pool supply stores or home improvement stores. I buy a 30% HCl solution at my local pool store (2 gal = $25) that routinely titrates to a 10 M concentration. This solution is somewhat yellow, but the yellow color is almost undetectable when diluted to 1 M, 2 M, or 3 M concentrations. 6 M will still be somewhat yellow. Instructor's Lab Set-Up: o Prepare a large volume of 2-M HCl (at least 50 mL per lab group) o Prepare volumes of 1-M, 3-M & 6-M HCl (25–50 mL per lab group) o Set out baking soda at weighing stations. Store adequate reserves so each group can use 25 grams of baking soda. o Provide a sampling of Styrofoam cups, metal cups, and ceramic cups for communal use on a "buffet table." Computer-linked thermometers recommended, so that the results of experiments are recorded for teacher review. The curvature of the temperature vs. time graphs are also valuable to stimulate student analysis of the results (e.g. temperature rising at the end of the graph indicates heat is being transferred into solution). If done with traditional thermometers, instructor should check to verify temperature results. Suggested grading rubric Lowest temperature Grade (out of 10 points) 7 Less than 1 C 7.5 Less than 6 C 8 Less than 10 C 8.5 Less than 12 C 9 Less than 14 C 9.5 Less than 20 C Best in the Class 10 FOR THE STUDENT Student Activity Sheet: Less than Zero Lab Lesson Background The objective in this lab is to cool an aqueous solution to less than 0 oC. You will first run a scripted reaction. Each group will then change variables and do subsequent trials to make the temperature drop further below 0 oC. Everyone will turn in an official note page at the end of the investigation. Procedure (scripted reaction) 1. Mass a 250-mL beaker. Record its mass. 2. Obtain a computer-interfaced thermometer (including USB link) and plug it into a computer. When prompted, choose Launch Data Studio. You should see a graph appear that has temperature on the y-axis and time on the x-axis. 3. Double click the Digits option on the lower left of the Data Studio screen to provide a box that easily allows you to read the temperature (with one or two decimal places). 4. Click the Run button to verify that your thermometer is working properly. Then click Stop to terminate this experiment. 5. Mass between 2.80 and 3.20 grams of baking soda (NaHCO3) on weighing paper. Record the exact mass of the sample. 6. Pour 25 mL of 2-M HCl into the beaker. Click Run to measure the initial temperature of the acid (make sure the temperature probe is in the acid). 7. With the thermometer recording data on the computer screen, carefully add the baking soda to the beaker (Warning: fizzing will occur). Gently stir the reaction with the thermometer and record data until the reaction is complete. 8. Right-click on the computer graph and choose Statistics to display the minimum temperature the reaction reached. 9. Record the mass of the beaker and its contents. Data Mass of beaker Mass of baking soda Volume and molarity of acid Lowest Mass of Temperature beaker and contents Calculations The reaction that occurred is: NaHCO3 (s) + HCl (aq) NaCl (aq) + H2O (l) + CO2 (g) ΔH = +28 kJ/mol 1. Calculate the number of kJ absorbed by the reaction assuming the baking soda was the limiting reactant. 2. Convert the kJ into calories. 3. Divide the calories by the number of grams of solution you have in your beaker. This will give you a theoretical value for how much your temperature should have dropped during the reaction. 4. Compare your theoretical temperature drop from with the experimental temperature drop you recorded. Ponder why these numbers are not the same. 5. Calculate the moles of HCl present in the experiment. Was it correct to assume that the baking soda was the limiting reactant? Analysis (further trials) Now you will modify the experiment to achieve an even colder temperature than you did in Part I. You have two attempts to get the temperature to drop as low as possible. The questions below are designed to help you consider possible modifications. These are NOT the only things that you could change. The best strategy will be to discuss the parameters you would like to change (and why) prior to performing actual experiments with your lab group members. Some things to consider: Does the container affect the results of the experiment? Ceramic, metal and Styrofoam vessels are available. Are the amounts of baking soda and acid that you used in the first reaction the “right" amount? Would there be an advantage to adding some extra water to the reaction? Is there an advantage to scaling up or scaling down the reaction? Is the volume of acid important? If so, do you want more or less volume in your reaction? Would there be an advantage to using a different molarity of acid? 1 M, 2 M, 3 M, and 6 M HCl are available Once you’ve decided what variables to change, complete the reaction. Depending on your results from that trial, you may want to consider again what modifications to make for your final trial. Trial #1: Type of reaction vessel used: ________________________________________ Mass of Moles of baking soda baking soda Volume and molarity of acid Moles of acid Lowest Temperature Theoretical ΔT What changes did you make to the original procedure? Why?? Trial #2: Type of reaction vessel used: ________________________________________ Mass of Moles of baking soda baking soda Volume and molarity of acid Moles of acid Lowest Temperature Describe the reasoning behind the modifications you made in this trial. Theoretical ΔT