Lecture thirteen-------------------------------------------------------------
advertisement
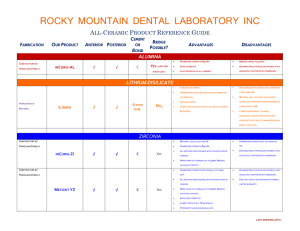
Lecture thirteen--------------------------------------------------------------crown and bridge احمد غانم.د Dental porcelain The term ceramic is defined as any product made essentially from a nonmetallic material by firing at a high temperature to achieve desirable properties. The term porcelain refers to a family of ceramic materials composed essentially of kaolin, quartz, and feldspar, also fired at high temperature. Dental ceramics for ceramic-metal restorations belong to this family and are commonly referred to as dental porcelains. The laboratory portion of a ceramic restoration is usually made in a commercial dental laboratory by a skilled technician working with specialized equipment to the shape and shade specifications provided by the dentist. Skilled technicians and artisans are also employed by the manufacturers of artificial denture teeth to produce the many forms, types, and shades necessary in this application of porcelain. Dental ceramics were first used in dentistry in the late 1700s. Porcelain jacket crowns were developed in the early 1900s. They consisted of feldspathic or aluminous porcelain baked on a thin platinum foil and can be considered the ancestors of all-ceramic crowns. Because their low strength, however, porcelain jacket crowns were limited to anterior teeth. In the 1960s, the poor match in thermal expansion (and contraction) between framework alloys and veneering ceramics, which often led to failures and fractures upon cooling, stimulated the development of leucite-containing feldspathic porcelains. The problem was solved by mixing controlled amounts of high-expansion leucite with feldspar glass at the manufacturing stage. This allowed the adjustment of the coefficient of thermal expansion of feldspathic porcelains to very narrow specifications. This invention led to considerable improvement in the reliability of ceramic-metals and allowed ceramic materials to be 1 Lecture thirteen--------------------------------------------------------------crown and bridge bonded to a metal framework. During cooling, the thermal contraction of the metal framework is slightly higher than that of the veneering ceramic, thus placing the internal surface of the ceramic in compression. Because ceramics are stronger in compression than in tension, this property is used to advantage to provide increased resistance to shattering. Classification of dental ceramics: Dental porcelains are classified into four groups: 1. High fusing porcelain (1300-1370 ºC). 2. Medium fusing (1100-1250 ºC). 3. Low fusing (850-1100 ºC). 4. Ultra low fusing < 850ºC. The high and medium fusing porcelain are used for denture teeth, while the low and ultra low fusing is used for crown and bridge construction. Composition: 1. Feldspar. 2. Quartz. 3. Kaolin. 4. Pigments and flux. 1. Feldspar: It’s the main component of the porcelain about 80% by weight. It’s a mixture of potassium alumino silicate (K2O.AL2O3.6SIO2) and sodium alumino silicate (Na2O.Al2O3.6SiO2). The feldspar fused when it melts forming translucent glass matrix. 2 Lecture thirteen--------------------------------------------------------------crown and bridge 2. Quartz: It forms about 15%, it acts as strengthening agent and present as fine crystalline dispersion throughout the glossy phase that is produced by the melting of the feldspar. 3. Kaolin: It’s about 4%, it’s a hydrated aluminum silicate (Al2O.2SiO2.2H2O) act as a binder and increasing the ability to mold the unfired porcelain and aid in forming a workable mass of porcelain during molding, it’s opaque in color. 4. Pigments and flux: It represents less than 1%; some coloring pigments (metallic oxide) are added in small quantities to provide wide variety of colors, e.g. iron oxide (brown shade), titanium oxide (yellow shade), and cobalt oxide (blue shade). Fluxes (low fusing glass) added to reduce the temperature that is required to set the porcelain powder particles at low temperature enough, so that the alloy to which it is fired, do not melt or get deform. APPLICATIONS: Ceramics have three major applications in dentistry: (1) ceramics for metal crowns and fixed partial dentures. (2) all-ceramic crowns, inlays, onlays, and veneers, when esthetics is a priority, (3) ceramic denture teeth. 3 Lecture thirteen--------------------------------------------------------------crown and bridge Bonding between ceramic to metal.: 1. Mechanical bond: By mechanical interlocking of porcelain with the roughness of metal coping (sandblasting the metal surface with aluminum oxide particles). 2. Vander Wall’s bonding: It’s the attraction between the atoms or molecules, it is adhesion related to the extent to which the metal is melted by the porcelain, the better wetting, the stronger the Vander Wall’s adhesion (bond). 3. Compression (physical bond): The coefficient of thermal expansion for the metal should be slightly higher than that of porcelain, so that the porcelain will draw toward the metal when the restoration cools after firing. 4. Chemical bonding: Metal and porcelain reacts chemically in an oxidized atmosphere at approximately 1000ºC to bond together (indicated by the formation of an oxide layer on the metal). The fused porcelain absorbs ions from the metal to produce a chemical bond between them. *** 20% of the bond is due to the first three factors, and 80% of the bond is due to chemical bond. Porcelain is built in three layers: 1. Opaque layer: it is the first layer applied on the metal to mask the color of the metal and it is responsible for the metal ceramic bond. 2. Dentine layer. 3. Incisal layer: this is translucent and affected by the color of the dentine layer. 4 Lecture thirteen--------------------------------------------------------------crown and bridge The powder is mixed with water and binder (which will help to held the particles together) and applied to the die either by spatulation, rush application, whipping or vibrating. The objectives of these techniques are to remove as much water as possible resulting in a more compact arrangement with a high density of particles which minimize the firing shrinkage. The thermal contraction of porcelain will be resisted by the metal and a compressive stress will be setup in the porcelain and will be firmly bonded to the metal. Properties of porcelain: 1. Esthetic: it has better look than acrylic (looks like vital tooth). 2. Color stability: glazed porcelain will provide a smooth surface which not allow adherence of stains, while the color of acrylic facing will change with time due to the porosity and rough surface of acrylic material. 3. Dimensional stability: the thermal coefficient and conductivity is close to that of the tooth structure that is why the microleakage is less likely to be a problem in the presence of good marginal seal, while in acrylic there is more microleakage because of the higher coefficient of thermal expansion than that of the teeth. 4. Irritation of the soft tissue: glazed porcelain close to gingival tissue will not cause inflammation because it’s very smooth which prevent plaque accumulation while the acrylic will cause gingival inflammation due to its porosity. 5. Bonding: the bonding between the porcelain and the metal is strong because it’s (mechanical, physical and chemical in nature)while the acrylic is mechanically bonded to the metal (by creating a rough surface with loops and beads). 5 Lecture thirteen--------------------------------------------------------------crown and bridge 6. Abrasion and wear resistance: glazed porcelain has high resistance to abrasion, while the acrylic facing will be abraded with time. The compressive strength of dental porcelain is high (350-550 MPa) while tensile strength is low (20-60 MPa) so the porcelain is hard but brittle. 7. Staining and glazing: stain can be applied to porcelain which can be glazed to give highly smooth surface. 6