Unit 7: Acid-Base Chemistry Content Outline: Defining Acids (7.1
advertisement

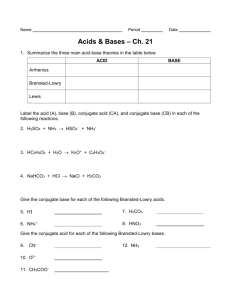
Unit 7: Acid-Base Chemistry Content Outline: Defining Acids (7.1) I. Acid A. An acid refers to a molecule in one of 3 ways: 1. Arrhenius Acids a. This method of defining an acid was proposed by the Swedish Chemist Svante Arrhenius in 1854. He also was the winner of two Noble Prizes for Chemistry – 1903 & 1905. b. This definition of an acid states that “A chemical compound that increases the Hydrogen Ion (protons)concentration [H+] in an aqueous solution is an acid.” i. The solute ionizes in the solvent to produce mobile protons [H+] c. These solutions are referred to as aqueous acids. i. They are electrolyte solutions as they have mobile protons [H+]. ii. They have a strong ability to form Hydronium Ions [H3O+] by combining with water. 2. Brønsted-Lowry Acids a. This method of defining an acid was proposed by Danish Chemist J.N. Brønsted and English Chemist T.M. Lowery in 1923. b. This definition of an acid states that “ A molecule or ion that is a proton [H+] donor is an acid.” i. Monoprotic Acids α. These donate a single (mono) proton. For example, HCl or HBr. Or HI ii. Polyprotic Acids α. These donate more than one (poly) protons. For example, H2SO4 or H3PO4 β. Because of the multiple proton donations, there can be multiple forms of the acid present at the same time in the same solution. For example, H3PO4 or H2PO4 or HPO4 or H+ The first ionization, H2PO4, is greatest in terms of presence (concentration). γ. These produce very interesting titration curves. You will talk about these more in depth in coming outlines. 3. Lewis Acids a. This method of defining an acid was proposed by the American Chemist G.N. Lewis in 1923. b. This definition of an acid states that “An atom, ion, or molecule that accepts an electron pair to form a covalent bond is an acid.” i. The product of the bonding is called an adduct. (Sounds like Add + product together) c. This method, unlike the two above does not require the presence of Hydrogen. For example, BF3 + F_ BF4_ c. Lewis acids must have 3 valence electrons (trigonal planar) with an empty p orbital. d. They usually are an element (x) paired with 3 halides (R) (Group 17). Expressed as XR3. e. The formation of an adduct may violate the Octet rule, but usually not. For example, I2 + I- I3_ i. This is referred to as Expanded Octet or Hypervalence.