Detailed Methods for Modeling Studies of Drosophila Integrins
advertisement

Detailed Methods for Modeling Studies of Drosophila Integrins Kishore Shakalya, Daruka Mahadevan Homology models of PS2m8 and PS2C were generated by threading fly and mosquito sequences onto the X-ray crystal structure coordinates of human v3 (1JV2) (Xiong et al., 2001). Various models were built including some comprising the whole protein, the -subunit -propeller domain alone, or this in combination with the -subunit A/I domain. Modeller (version 7) was used for homology modeling of aligned sequences and then superimposed (homology aligned using Sybyl 7.2) on the original crystal structure and merged into a single coordinate file after removing the v structural coordinates. The models were energy minimized using Sybyl 7.2. For PS2C, the mosquito sequence was done intially, and the fly structure was then modeled after this. This sequential approach was taken because of problems caused by the proline rich loop in the fly structure. For Anopheles gambiae PS2 no cDNA sequence was available. The gene was found by searching the An. gambiae genome for the PS2 homolog, constructed by virtual cDNA identification for each exon and analyzing proposed splicing sequences in detail. The locations and reading frames of the relevant splice sites are identical to those in Drosophila. The homology model built by Modeller (version 7) included exon 8 region insertion of amino acid residues between Gln193 and Gly219. 194 GQTYSIPPDAKFPFKPPLYQPFGTG 218 This insertion is proline rich and was built by Modeller (version 7) as a distorted helix. In a given protein structure the backbone Catoms serve as the corner attachment point of two different planes, each which can rotate independently of the other plane. The two planes can twist around the C carbon. The only degrees of freedom along the backbone are about the NH-C bond called the phi and the C – C'O bond called the psi angle. They can vary from -180o to +180o. Since proline rich regions are not -helical we checked the phi-psi angles of the residues in the inserted exon 8 segment. The ideal bond angles for an extended collagen-like poly-proline helix are phi = -70o and psi = +138o respectively. These angles were measured using Sybyl 7.2. It was found that the 4 prolines were modeled with phi/psi angles that produced a distorted -helix. Residues Phi angle Psi angle Pro 201 -48.97 -34.81 Pro206 -56.49 -31.37 Pro210 -86.69 49.83 Pro214 -42.14 -39.51 To change the phi-psi angles, one residue on either side of each proline was extracted and the phi-psi angles were changed to match an ideal collagen-like polyproline helix. A local energy minimization was performed for a region of around 5Å incorporating the four prolines in Sybyl 7.2. The final model was superposed on the original model to obtain a root mean square deviation of <1Å. The phi-psi angles were then measured after the changes were done. As seen in the table below, they closely conform to the ideal case. The minor difference in the angles as compared to the ideal case can be attributed to the surrounding residues and the overall shape of the structure. Residues Phi angle Psi angle Pro 201 -47.45 138.68 Pro206 -56.56 158.07 Pro210 -69.27 157.68 Pro214 -37.01 114.26
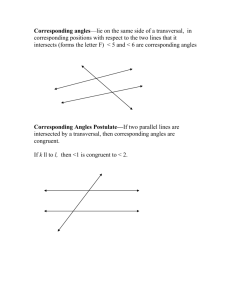

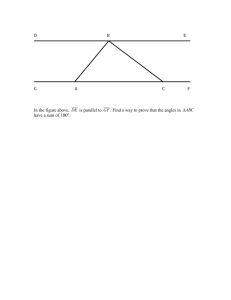

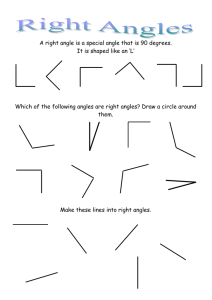


![7th Grade [Pre-Algebra] Math Vocabulary](http://s3.studylib.net/store/data/006617991_1-76ce0e26cff8b794b821343c050f71cb-300x300.png)

