Bioassay Guided Isolation, Structure Elucidation and Synthesis of
Bioassay Guided Isolation, Structure Elucidation and Synthesis of Neuroactive
Alkaloids from Plants, Amphibians and Arthropods.
The plant kingdom has for millenia been a treasure trove of compounds for the treatment of disease. Although in recent years the animal kingdom has been explored for medicinals, plants remain the major source of bioactive compounds. Screening of libraries from combinatorial chemistry programs has produced significant successes, but random screening of synthetic compounds has limited value in a directed target oriented campaign. Screening natural products, on the other hand, starts from a bioactive position.
Nature rarely, if ever produces a compound without a defined biological purpose aimed at a survival advantage. Chemists and pharmacologists have long recognized this fact and mined the natural world for interesting structures and activities.
Traditionally, natural products have first been isolated from a crude extract and structurally characterized prior to analysis of bioactivity. Otherwise the bioactivity of the crude extract has been assessed at some concentration and ruled active or otherwise based on a rough "combinatorial" screen, wherein interfering activities can cancel each other out. Thus, many potentially useful compounds may have been overlooked due either to such interference or insufficient material for full structural characterization given the analytical technology of the time.
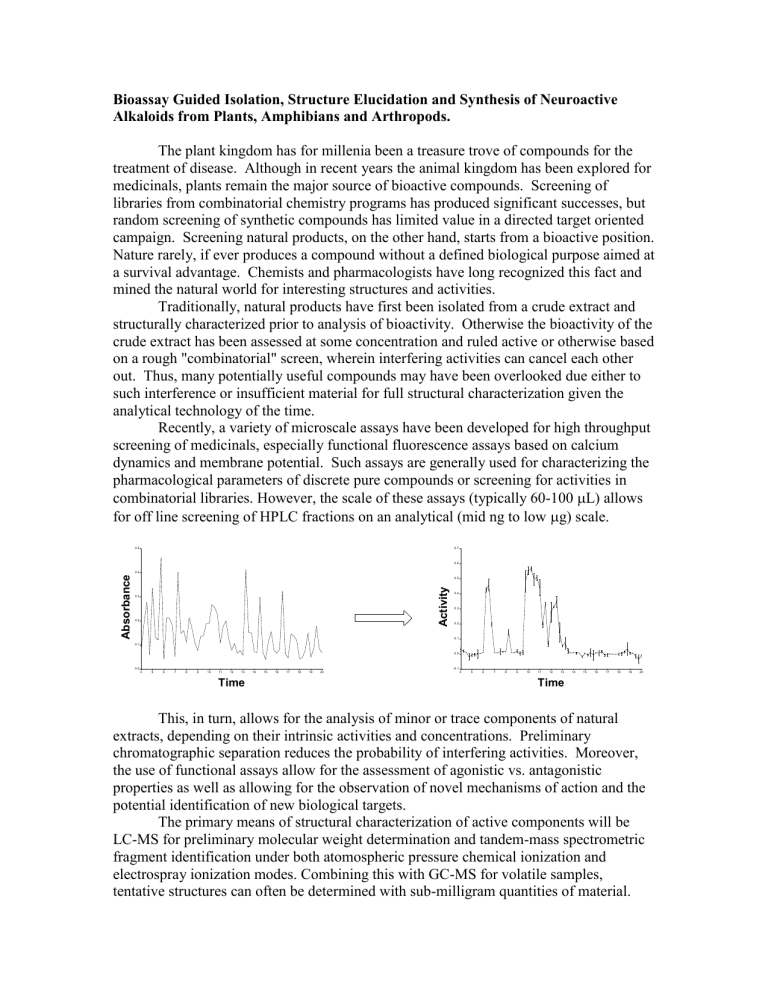
Recently, a variety of microscale assays have been developed for high throughput screening of medicinals, especially functional fluorescence assays based on calcium dynamics and membrane potential. Such assays are generally used for characterizing the pharmacological parameters of discrete pure compounds or screening for activities in combinatorial libraries. However, the scale of these assays (typically 60-100
L) allows for off line screening of HPLC fractions on an analytical (mid ng to low
g) scale.
0.5
0.7
0.4
0.3
0.2
0.1
0.6
0.5
0.4
0.3
0.2
0.1
0.0
0.0
4 5 6 7 8 9 10 14 15 16 17 18 19 20
-0.1
4 5 6 7 8 9 10 11 12 13
Time
14 15 16 17 18 19 20 11 12 13
Time
This, in turn, allows for the analysis of minor or trace components of natural extracts, depending on their intrinsic activities and concentrations. Preliminary chromatographic separation reduces the probability of interfering activities. Moreover, the use of functional assays allow for the assessment of agonistic vs. antagonistic properties as well as allowing for the observation of novel mechanisms of action and the potential identification of new biological targets.
The primary means of structural characterization of active components will be
LC-MS for preliminary molecular weight determination and tandem-mass spectrometric fragment identification under both atomospheric pressure chemical ionization and electrospray ionization modes. Combining this with GC-MS for volatile samples, tentative structures can often be determined with sub-milligram quantities of material.
Where sufficient quantities of purified material are available, NMR and x-ray crystallographic analysis would be used to confirm the structure. In the absence of sufficient isolates, however, larger quantities of plant extracts could be processed to isolate the bioactive material in workable amounts. In either event, synthesis and side by side comparison of pharmacology is then appropriate, as in many cases the major compounds co-eluting with activities are not responsible for the activity. Rather, a minor or trace impurity is often responsible.
The analysis of domestic plants is of practical concern. While examination of extracts from exotic foreign sources has a certain aesthetic appeal, the advent of restrictions on foreign bioprospecting, the expense and logistical issues involved in international collecting can make such endeavors somewhat impractical. Moreover it is my firm belief that the plants in our own backyard are an underutilized resource. While taxol is available from the yew, such plants are more often used as landscape ornamentation than as the source of medicines. Those which have been examined for structurally or biologically interesting compounds often were studied at a time when the sensitive analytical methods we have today did not exist. Thus it is likely that many bioactive components have been overlooked.
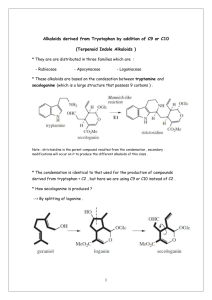
One of the first places we are looking is in various members of leguminosae (pea family). The alkaloids derived from these plants have been extensively studied and reviewed from a chemical standpoint and the toxicity of members of this family are well documented, especially those of the lupins. Its toxic principles are considered to be the quinolizidine alkaloids. Cytisine, sparteine and several other of the quinolizidine alkaloids are active at nicotinic and/or muscarinic acetylcholine. Anagyrine has been implicated as a teratogen, leading to crooked calf disease. Bitter lupins have been implicated in many cases of ruminant intoxication, leading to reduced productivity and in some cases death of livestock.
NH N
N N
O
Cytisine
O
Anagyrine
N
N
Sparteine
The rationale for the choice of lupins is threefold. First, neurological activities have already been ascribed to some of the chemical constituents of these plants, thus there is a high probability of finding further active constituents. Such cholinergically active compounds have value as analgetics, and potential therapeutics for Parkinson's disease, Alzheimer's disease, and Tourette's syndrome, as well as utility as pharmacological probes. Second, most of the major and minor lupine alkaloids have been
structurally characterized and many have been synthesized, thus known components can be readily identified and reference natural and synthetic materials can be evaluated for activity to confirm or refute activities initially ascribed to these components. Third and finally, lupins are readily accessible, growing wild in the US, thus collection of samples for workup are not difficult to obtain in quantities sufficient for analysis of minor and trace components. Other members of the family such as the brooms and laburnum are common ornamentals and herbals and are also readily obtained.
Depending on the success of this project, further projects are envisioned along similar lines with members of Nicotiana , the family containing nicotine, as well as cultures of Anabena flos aquae , the dinoflagellate from which (+)-anatoxin is derived.
H
N
COMe
N
CH
3 N
(-)-Nicotine (+)-anatoxin-a
A standing collaboration with researchers at the National Institutes of Health is concerned with the identification of novel activities in the skins of amphibians, especially dendrobatid frogs from the new world tropics, as well as pseudophryne and mantellid frogs from Australia and Madagascar, respectively. As it has been shown that in the majority of these frogs (except pseudophryne) the alkaloids in their skins are derived from their diets, arthropod collections are also being examined in order to identify the source of the alkaloids, many of which have not been previously observed in arthropods.