Example No
advertisement
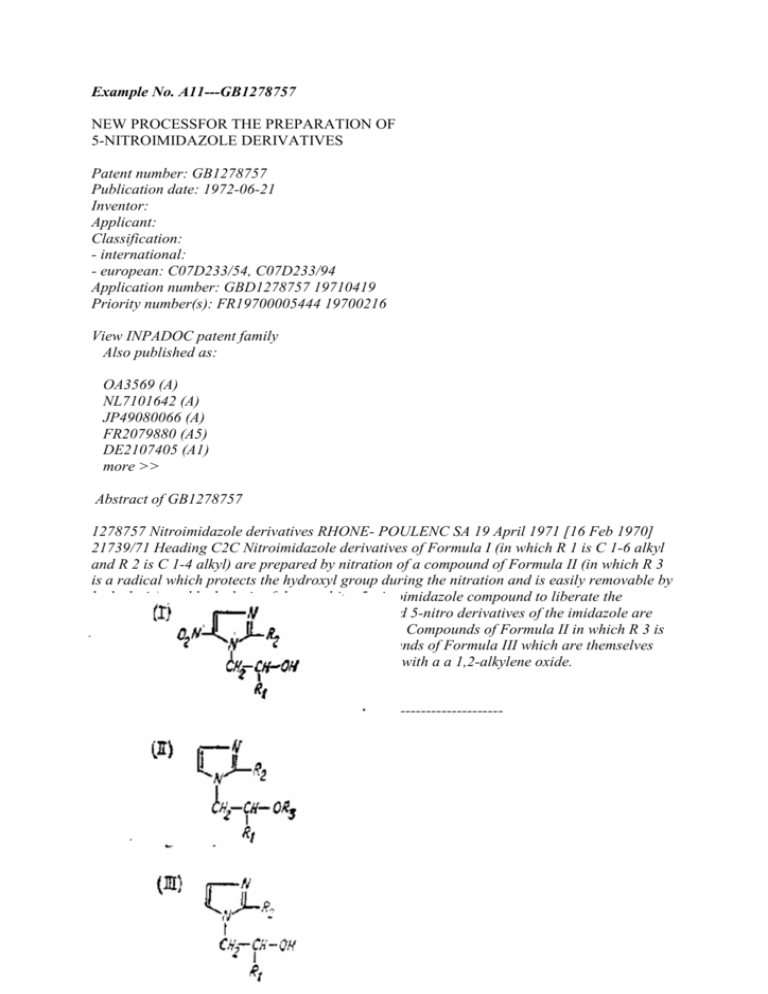
Example No. A11---GB1278757 NEW PROCESSFOR THE PREPARATION OF 5-NITROIMIDAZOLE DERIVATIVES Patent number: GB1278757 Publication date: 1972-06-21 Inventor: Applicant: Classification: - international: - european: C07D233/54, C07D233/94 Application number: GBD1278757 19710419 Priority number(s): FR19700005444 19700216 View INPADOC patent family Also published as: OA3569 (A) NL7101642 (A) JP49080066 (A) FR2079880 (A5) DE2107405 (A1) more >> Abstract of GB1278757 1278757 Nitroimidazole derivatives RHONE- POULENC SA 19 April 1971 [16 Feb 1970] 21739/71 Heading C2C Nitroimidazole derivatives of Formula I (in which R 1 is C 1-6 alkyl and R 2 is C 1-4 alkyl) are prepared by nitration of a compound of Formula II (in which R 3 is a radical which protects the hydroxyl group during the nitration and is easily removable by hydrolysis), and hydrolysis of the resulting 5-nitroimidazole compound to liberate the hydroxyl group. During the nitra- tion, 4-nitro and 5-nitro derivatives of the imidazole are formed and these are separated by usual methods. Compounds of Formula II in which R 3 is alkanoyl are prepared by esterification of compounds of Formula III which are themselves obtained by reaction of a 2-C 1-4 alkyl-imidazole with a a 1,2-alkylene oxide. -------------------------------------------------------------------------------- Description of GB1278757 (5T) NEW PROCESSFOR THE PREPARATION OF 5-NITROIMIDAZOLE DERIVATIVES (71) Wc, RHONE-POULENC S.A., a French Body Corporate of 22, Avenue Montaigne, ParisSe, France, do hereby declare the invention for which we pray that a patent may be granted to us, and the method by which it is to be performed, to be particularly described in and by the following statemeat:This invention relates to a new process for the preparation of 5-nitroimidazole derivatives of the generalformula:: EMI1.1 wherein <RTI R1 represents astraight-or branchedchain alkyl group containing 1 to 6 carbon atoms (preferably methyl orethyl), 5lnd R represents a straight- or branched-chain alkyl group containing 1 to 4 carbon atoms (preferably methyl or ethyl), which possesschemo- therapeutic properties; in particular they are active as anti-protozoal agents andespecially as amoebicides and trichomonacides. In the specification of our British Patent No. 1079271 entitled " ImidazoleDerive- tices" and granted on an application filed Oct. 8, 1964, there are described and claimed inter also 5-nitroimidazoles of the foregoing formula and two processes for their preparation, one process involving (a) the reaction in the absence of a basic condensing agent of a nitroimidazole of the generalformula: EMI1.2 (wherein2 is as hereinbefore defined) with a reactive ester reactant of theformula:: EMI1.3 (wherein Z represents the acid residue of a reactive ester, Y represents a hydrogen atom or a radical which protects the hydroxyl group during the reaction and is readily replaceable by a hydrogen atom, and <RTI Rl is as hereinbefore defined) and, where Y is other than a hydrogen atom, liberation of the hydroxyl group by methods known perst, and the other process involving (b) the reaction of a nitroimidazole of general formula II with a 1,2-alkyleneoxide of the generalformula: EMI1.4 (whereinRl is as hereinbefore defined) in a liquid medium containing or consisting of at least one organic acidwhich is a solvent for the nitroimidazole. It has now been found as a result of research and experimentation that the 5-nitroimidazole derivatives of general formula I can also be preparedb:r the newprocess which comprises the nitration of an imidazole derivative of the general formula: EMI2.1 (whereinRl andR are as hereinbefore defined andR represents a radical which protects the hydroxyl group during the nitration reaction and is easily removable by hydrolysis) bv known methods for the nitration of imidazoles, and hydrolysis of the resulting 5-nitroimidazole compound to liberate the hydroxyl group.The radicalR!, besides being easily removeable byhydrolysis, must also be stable under the conditions of nitration of the imidazole starting material. Suitable groupsmee.- ing these requirements are, for example, alkanoyl radicals containing 1 to 4 carbon atoms, in particularacetyl. Nitration of the imidazole starting materials of general formula V is preferably effected with nitric acid in the presence of phosphorus pentoxide, advantageously at a temperature below10 C. Hydrolysis of the 5-nitroimidazole products is preferably effected in an acid medium (e.g. hydrochloric acid), advantageously at atemperature of 90 -100 C. During the nitration, 4-nitro and 5-nitro derivatives of the imidazole of general formula V are formed and these isomers can be separated, either before or afterhydrolysis, by the chemical or physico-chemical methods usually employed for the separation of positonally isomeric nitro derivatives of imidazole, such as fractional crystallisation or columnchromatography. Theimidazoies of generalformula V ill'-ghich R3 represents analkanoyl radical can be obtained by esterificationbv known methods of an imidazole derivative oftke general forr.lula: EMI2.2 v) R@ and R@ are as hereinbefore de- fined.pro l'ly, an alkanoylchloride in an organic solvent, for example acetonitrile, is used at the reflus temperature of the reaction mixture. The compounds of general formula VI can be obtained in accordance with known methods forN-hydroxyethylation of the imidazole ring. In particular, they can be obtained by the action of an epoxide of general formula IV on an imidazole derivative of the general formula: EMI2.3 whereinIt is as hereinbefore defined. The 5-nitroimidazole derivatives ofgeneral formula I obtained according to the process of the present invention can optionally be purified by physical or chemical methods and can be convertedbv known methods into acid addition salts. By theterm "known methods" as used in this specification is meant methods heretofore used or described in the chemical literature. The following Example illustrates the process according to the invention. EXAMPLE 1 - (2 - Acetoxypropyl) -2 - methylimi- dazole (18.2 g.) is gradually dissolved in fuming nitric acid(d=1.52; 25 cc.) with stirring, the temperature being kept at about 2 C. Phosphorus pentoxide (20 g.) is added, with caution, to the resulting solution and whilst maintaining the temperature at about2 C. Afterwards, the reaction mixture is stirred for a further 3 hours 30 minutes at2 C. and poured onto ice (180g.). The solution obtained is treated with ammo Ilium hydroxide (d=0.92;105 cc.), saturated with sodium chloride, and then extracted with ethyl acetate (total 650 cc.). The combined organic extracts are washed with a saturated aqueous sodium chloride solution (50 cc.) and then dried over sodium sulphate. The volatile products are evaporated under reduced pressure (20 mm.Hg.) and a mixture of 1 (2 acetoxypropvl) - 2 - methyl - 4 - nitroimidazole and 1 - (2 - acetoxypropyl) - 2 methyl - 5 nitroimidazole (18.6 g.) is obtained in the form of a red oil. A solution of a mixture of 1 - (2 -acetoxy- propyl) - 2 - methyl - 4 - nitroimidazole and of1 (2 - acetoxypropyl) - 2 - methyl - 5 nitroimidazole (18.6 g.) [prepared as described above] in 4N hydrochloric acid (186 cc.) is heated at902 C. for 90 minutes. The cooled solution is treatedwith ammonium hydroxide (d=0.9; 100 cc.), saturated with sodium chloride, and then extracted with ethyl acetate (total 550 cc.). The combined organic extracts are washed with a saturated aqueous sodium chloride solution (50 cc.) and then dried over sodium sulphate. The volatile products are evaporated under reduced pressure (25 mm.Hg.); the residual brown oil weighs 9.2 g. This oil (5.8 g) is dissolved in methyl ethyl ketone (20 cc.) and chromatographed over silica (232 g.) contained in a column 4.5 cm. in diameter. The column is eluted with methyl ethyl ketone; the first 600 cc. of eluate are discarded and 500 cc. of eluate are then collected and concentrated under reduced pressure (25 mm.Hg.); a partially crystalline product (2.4 g.) is thus obtained. 1 - (2 - Hydroxypropyl) 2 - methyl - 5 - nitroimidazole (0.96g.), m.p.72" C., is obtained on recrystallisation from water (4 cc.). The 1 - (2 - acetoxypropyl) - 2 - methylimidazole employed as starting material can be obtained by heating a mixture of 1 - (2 hydroxypropyl) - 2 - methylimidazole (35 g.) and acetyl chloride (23.6 g.) in acetontrile (350 cc.) under reflux for 3 hours 30 minutes. Thereafter the volatile products are evaporated under reduced pressure (25mm.Hg), the residue is taken up in water (50 cc.) and ethylacetate (250 cc), and the mixture is then rendered alkaline to pH 10 by addition of 10N sodium hydroxide solution the temperature being kept below20 C. The organic phase is decanted and the aqueous phase is then extracted with ethyl acetate (200 cc.). The organic extracts are combined and then dried over anhydrous sodium sulphate. The solvents are evaporated under reduced pressure (25 mm.Hg) and 1 - (2 - acetoxypropyl) 2 - methylimidazole (35.3 g.) is obtained in the form of a brown oil. 1 - (2 - Hydroxypropyl) - 2 - methylimidazole can be prepared by adding propylene oxide (94.5 g.) to 2 - methylimidazole (82 g) dissolved in ethanol (330 cc.). The reaction mixture is left at a temperature of about20" C. for 72 hours and then concentrated under reduced pressure (25 mm.Hg). Distillation of the residue under reduced pressure (0.4 mm.Hg) yields 1 - (2 - hydroxypropyl) 2 - methylimidazole (137.2 g.), b.p.144" C. WHAT WE CLAIM IS:1. Process for the preparation of 5-nitroimidazole derivatives of the general formula: EMI3.1 (whereinRl represents a straight- or branchedchain alkyl group containing 1 to 6 carbon atoms, andIt. represents a straight- or branched-chain alkyl group containing 1 to 4 carbon atoms) which comprises the nitration of an imidazole derivative of the generalformula: EMI3.2 (wherein <RTI Rl and R, are as hereinbefore defined, andRa represents a radical which protects the hydroxyl group during the nitration reaction and is easily removable by hydrolysis) by known method for the nitration of imidazoles, and hydrolysis of the resulting 5 nitroimidazole compound to liberate the hydroxyl group. 2. Process according to claim 1 wherein R3 represents an alkanoyl radical containing 1 to 4 carbon atoms. 3. Process according to claim 1 or 2 whereinRg represents the acetyl radical. 4. Process according to claim 1, 2 or 3 in which nitration of the imidazole ring is effected with nitric acid in the presence of phosphorus pentoxide. 5. Process according to claim 4 in which the nitration is carried out at a temperature below10 C. 6. Process according to any one of the preceding claims in which hydrolysis of the 5nitroimidazole intermediate compound is effected in an acid medium. 7. Process according to claim 6 in which the hydrolysis is effected at90"--100" C. 8. Process according to any one of the preceding claims in whichR1 andR, in the general formulae specified in claim 1 each represent a methyl or ethyl group. 9. Process for the preparation of 5-nitroimidazole derivatives of the general formula specified in claim 1 substantially as described in the foregoing Example. 10. 5 - Nitroimidazole derivatives of the general formula specified in claim 1 when prepared by the process claimed in any one of claims 1 to 9.


![[VO(H2O)5]H[PMo12O40]-catalyzed nitration of alkanes with nitric acid](http://s3.studylib.net/store/data/007395962_1-c5684ccdbf5a6a8d13576cb676ea7c0b-300x300.png)




