Field Studies - Southern Connecticut State University

Protocol #
Submission Date
Review Date
Approval Date
P&D level
Southern Connecticut State University
REQUEST FOR USE OF VERTEBRATE ANIMALS IN FIELD STUDIES
Principal Investigator:
Office Phone:
Email address:
Department:
Lab Phone:
Emergency #:
Title of Protocol:
Anticipated beginning and ending dates of animal studies described in this protocol:
Check if animals will be used in:
Undergraduate honors thesis project or independent study
Graduate thesis project or independent study
Faculty research project
Other Explain
For animal use in research, specify funding source and project title. If the same research proposal involving the identical animal use will be submitted to more than one funding source, list the additional funding source(s) and the project title(s):
Are funding sources internal (SCSU or CSU funds) or external?
Funding source(s) and Project Title(s):
Internal
External
Page 1 of 4
11 Dec 2015
I. OVERVIEW OF PROPOSED PROJECT
A.
Describe the goals and overall potential benefit(s) of the research to humans and/or animals and the advancement of science.
B.
Describe all observational procedures, any manipulations or interaction(s) with animals, and whether animals will be disturbed or affected being brief and specific. Indicate if federal, state and/or local permits are required and whether they have been obtained.
Use language appropriate to eighth grade lay person without scientific background. Note that field studies are conducted on wild animals in their natural habitat. Field studies do not involve procedures or methods that harm or otherwise alter the behavior of the animals under study. Field studies do not require animal confinement for 12 or more hours. All of these parameters must be satisfied in order for a study to be considered a field study.
C. Describe the characteristics of this animal species that justify its use in the proposed studies:
D. State the names of every individual involved in the animal component of these studies and describe the primary responsibilities of each individual.
For each person, include their background/education/experience with the species and the specific procedures proposed in the protocol.
Individuals can perform only the specific procedures for which they have documented competence.
E. Bibliography. Any style is acceptable as long as it is consistent, and a link to the body of work must be provided.
II.
DESCRIPTION OF ANIMAL SUBJECTS
A. Species:
Sex:
Estimated number of animals observed per year:
Maximum number housed at one time:
Strain/Breed:
Age:
B. Describe how the number of animals was determined:
Page 2 of 4
11 Dec 2015
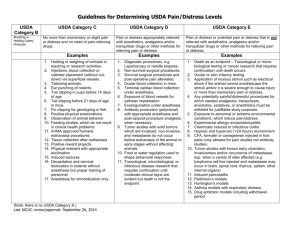
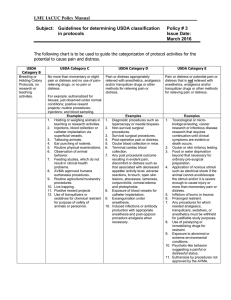
Pain and Distress Category.
Please indicate the level of pain and distress involved according to the following guidelines :
USDA Category “B”: This category is to be used for breeding or holding animals where no research is being conducted.
USDA Category “C”: Procedures, routine injections of non-toxin, non-irritating substances or venipuncture that produces minimal, transient, or no pain or distress.
USDA Category “D”: These procedures would cause more than minimal or transient pain and/or
distress, but are performed using appropriate anesthetics, analgesics, or tranquilizers. Examples are survival and terminal surgery (including perfusion and biopsy). Induction of disease states or drug and radiation exposures may fall into this category.
USDA Category “E”: These procedures cause more than minimal or transient pain and/or distress but cannot be performed using anesthetics, analgesics or tranquilizers without adversely affecting the study. Mechanical restraint may, depending upon duration and type of restraint, be considered a Category “E” study. Approval to conduct Category “E” studies requires detailed scientific justification in a separate paragraph (see Section IV.). This information must be forwarded by
SCSU to the USDA (for USDA covered species only) as part of our Annual Report, and is accessible to the general public via the UDA Website in accordance with the Freedom of Information Act.
Category B
Category C
Category D
Category E
11 Dec 2015
Page 3 of 4
V. SIGNATURES
A. Certification by Principal Investigator or Faculty Sponsor:
I affirm that to the best of my knowledge, information provided in this Request for Use of Vertebrate
Animals is complete and accurate, and that no significant changes will be made without advance approval of the
Institutional Animal Care and Use Committee. I further certify that these studies do not unnecessarily duplicate previous experiments.
As Principal Investigator or Faculty Sponsor of this project, I understand and accept that I have primary responsibility for all facets of this research including assurance that all animals used in this project will be handled in a manner that is humane and in accordance with standards set forth in the Animal Welfare Act, the Guide for Care and Use of Laboratory Animals, Public Health Service Policy and all other laws, policies, and accreditation standards that pertain to humane care and use of laboratory animals. Further, I assure that all individuals using animals under the provisions of this protocol have been trained or will be trained to competently and humanely perform the procedures listed on the chosen species prior to handling any animals.
Principal Investigator or
Faculty Sponsor
Date
Student Investigator Date
B. Certification by Department Chair
Note: The departmental chair must approve this application by signing if animal research will use departmental or non peer-reviewed funding.
In signing this form, the Department Chair assures corporate approval of the animal studies contained herein and acknowledges that the proposed use of animal facility resources complies with the overall mission and objectives of his/her Department.
Department Chair Date
C. Approval Signatures
The undersigned have evaluated the care and use of animals described in this protocol in accordance with provisions of the Animal Welfare Act, the PHS Guide for the Care and Use of Laboratory Animals, and the U.S.
Interagency Research Animal Committee Principles for the Utilization and Care of Research Animals, and find the procedures described appropriate and acceptable. (Comments and dissenting views may be noted below the approval signatures.)
IACUC Chairperson Date
Institutional Veterinarian Date
Page 4 of 4
11 Dec 2015