hepatic metabolism of midazolam is inhibited in critically ill patients
advertisement

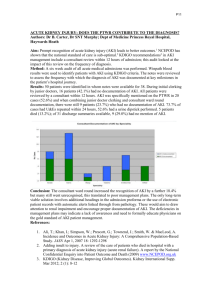
P108 HEPATIC METABOLISM OF MIDAZOLAM IS INHIBITED IN CRITICALLY ILL PATIENTS WITH ACUTE KIDNEY INJURY Kirwan. C1,3, Philips, B1, Lee, T2, Holt, D2, MacPhee, I3 1 Departement of Intensive Care, 2Analytical Unit, 3Department of Renal Medicine, St George’s University of London Chronic Kidney Disease (CKD) has been shown to cause a decrease in the hepatic metabolism of drugs in vivo and in vitro. The impact of acute kidney injury (AKI) on hepatic drug metabolism is untested. A single time point determination of midazolam concentration, four hours after intravenous administration, accurately predicts total midazolam exposure and thus metabolism in critically ill patients. Midazolam metabolism is a measure of hepatic activity of cytochromes P450 (CYP3A) 3A4 and 3A5 that are involved in the metabolism of >50% of widely used drugs. METHODS: All patients admitted to the adult intensive care unit were considered unless they had received a benzodiazepine in the previous 24 hours, had acute or chronic hepatic failure, were pregnant or were being administered major CYP3A inhibitors i.e. macrolide antibiotics or an imidazole anti-fungal. 1mg midazolam was given intravenously at time 0 and serum collected at 4 hours. Serum midazolam concentration was determined by mass spectrometry: T4[mid]. Patients were categorised using the AKIN / RIFLE criteria. Glomerular filtration rate was assessed by simultaneous 4 hour creatinine clearance (4CrCl) measurements. 120 ** T4 [mid] (ng.mL-1) 15 * *** 10 5 s) ) er tli 3 ou no 3 (F )( I( K A (F ( I) 2 ) (R 1 t li er ou no or m al (N ) s) 0 N RESULTS: 72 patients (53 with AKI) were recruited. Mean age 72 (23-90); 45 male. 19 had normal renal function with 16, 20, 17 in groups 1(R), 2(I) and 3(F) respectively. In critically ill patients the median (range) T4 [midazolam] concentration N v AKI was 3.15 (1.29-10.27) and 5.57 (0.59-113.6) ng.mL-1 respectively (p<0.005). There were two major outliers which may skew the results in favour of the hypothesis. If these two results are removed (Fig. 1) the statistical significance remains strong (N v * p<0.008; ** p=0.003; *** p=0.009)). Standard multiple regression analysis found the most useful predictors of T4[mid] were ‘time with AKI’ and ‘serum urea’ (beta coefficient 0.33 and 0.31 (p<0.05) respectively). 4CrCl, Serum creatinine and urine output did not add further predictive statistical power. CONCLUSION: This study demonstrates a reduction in the hepatic metabolism of midazolam associated with AKI. This effect is related most strongly to the length of time the patient has suffered with AKI.