Rock On - California Lutheran University
advertisement

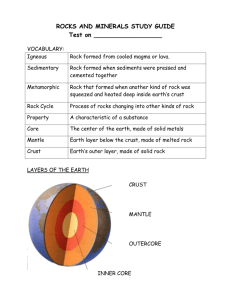
Rock On! A Hands-On Geology Unit Developed by: Robin Satnick Overview: This 10 session series encompasses a wide variety of hands-on lab experiments that explore and investigate rocks and minerals. This unit was written for 4th and 5th grade students who participate in an 1 ½ hour lab time once a week. This unit may easily be modified for older students. All units include background information, objectives, purpose, materials, worksheets for results and conclusions. Also included are excellent interactive websites to support lessons, reading book ideas and bibliography. The Ten Units are as follows: The Earth’s Layers Sedimentary Rock Igneous Rock Metamorphic Rock Rock Cycle Minerals Mystery Minerals Minerals for Health Volcanoes Earthquakes The California State Standards in Science was taken into consideration when developing these lessons. The following Standards are met for the 4th grade. Earth Science 4a. Students know how to differentiate among igneous, sedimentary and metamorphic rocks by referring to their properties and methods of formation (the rock cycle). 4b. Students know how to identify common rock-forming minerals (including quartz, calcite, feldspar, mica, and hornblende) and ore minerals by using a table of diagnostic properties. 5a. Students know some changes in the earth are due to slow processes, such as erosion, and some changes are due to rapid processes, such as landslides, volcanic eruptions, and earthquakes. 5b. Students know natural processes, including freezing and thawing and the growth of roots, cause rocks to break down into smaller pieces. 5c. Students know moving water erodes landforms, reshaping the land by taking it away from some places and depositing it as pebbles, sand, silt, and mud in other places (weathering, transport, and deposition). 6a. Differentiate observation from inference (interpretation) and know scientists’ explanations come partly from what they observe and partly from how they interpret their observation. 6b. Measure and estimate the weight, length, or volume of object. 6c. Formulate and justify predications based on cause-andeffect relationships. 6d. Conduct multiple trials to test a predication and draw conclusions about the relationship between predications and results. 6f. Follow a set of written instructions for a scientific investigation. The following standards are met for the 5th grade. 6a. Classify objects (e.g. rock) in accordance with appropriate criteria. 6b. Develop a testable question. 6c. Plan and conduct a simple investigation based on a student-developed question. 6g. Record data by using appropriate graphic representation (including charts, graphs and labeled diagrams.) 6h. Draw conclusions from scientific evidence and indicate whether further information is needed to support a specific conclusion. The Earth’s Layers Lesson I We walk on it, swim in its waters, and live on it. Planet Earth is an amazing composition of rocks, minerals, liquids and gasses! Today we will start our exploration of the rocks and minerals that make up the ground we walk on. Geology is the scientific study of the nature, formation, origin and development of the Earth’s crust and its layers. The Earth is made of four different layers. The crust of the Earth is like the skin of an apple. It is very thin in comparison to the other three layers. On top of the crust is the biosphere and this is where all living things dwell. The Earth’s crust is only about 3-5 miles thick under the oceans (oceanic crust) and about 25 miles thick under the continents (continental crust). The crust of the Earth is broken into many pieces called plates. The plates “float” on the layer below called the mantle. The mantle is composed of very hot, dense rock approximately 1800 miles thick making it the largest layer in the composition of the Earth. The mantle is made mainly of silicon, oxygen, aluminum and iron. The core makes up about 30% of the Earth’s mass. The outer core is made of liquid nickel and iron. The inner core is also made of nickel and iron and is under such great temperatures and pressures that the metals are in a solid state of motion. http://volcano.und.nodak.edu/vwdocs/vwlessons/lessons/Earths_layers/Earths_layer s2.html CLASSROOM DEMONSTRATION Apple of the Earth Objectives: 1. Students will be able to name and identify the four layers of the Earth. 2. Students will learn the size and composition of each layer. Materials: Apples Knife Procedure: 1. Cut enough apples into fourths so each student gets ¼ of an apple. 2. Have the students make observations of the apple. Tell the students that an apple is like the layers of the Earth. a. The skin of the apple represents the crust of the earth. The skin of the apple is very thin compared to the fleshy fruit. This is a good comparison to the earth’s crust mass compared to the mantle and core. b. The fleshy fruit or “meat” of the apple represents the mantle. The mantle is the largest layer of the Earth. The mantle is composed of molten rock that is in a semiplastic state. The composition is similar to very hot asphalt. c. The core of the apple represents the outer and inner core of the earth. The core is like a round ball in the middle of the Earth. The outer core is actually composed of very hot liquid nickel and iron and the inner core is made of the same elements but is in a solid state because of the intense pressure. Core Earth Samples- Cupcake Geology **It’s best to make these cupcakes ahead to save class time. Objectives: 1. To give students a visual and tactile experience of what the Earth’s interior is like. 2. Students will understand that geologist take core samples of the Earth in a similar fashion to our cupcake core samples. Materials: Chocolate cake mix Chocolate pudding Hard candies Paper plates Cupcake liners white cake mix toffee pieces green icing clear thick straws sprinkles (optional) Procedure: 1. Make the chocolate and white cake mixes. 2. Make chocolate pudding. 3. Place 24 liners in cupcake molds. 4. Layer the cupcakes – chocolate cake mix (core), pudding (outer core), white cake mix with candy pieces (mantle), green icing (crust), sprinkles (biosphere) Classroom Procedure with cooked cupcakes: 1. Have students use their straws to take “core samples of their cupcakes. Students need to gently push the straw through the cupcake. 2. Hold finger over the straw and gently pull up. 3. Blow through open end of the straw to remove cupcake from the straw. 4. Observe the different layers. LESSON II Sedimentary Rock Sedimentary rock makes up about three-quarters of the rocks on the earth’s surface. Sedimentary Rock is made up of materials that were once a part of another rock. These parts, called sediments, were transported by water, wind or glaciers and formed in layers. They form at the surface in environments such as beaches, rivers, oceans and any where there is sand, mud, and other types of sediments that collect. Over time, these loose sediments compress and form larger rock formations. Sedimentary rocks preserve a record of the environments that existed when they form. By looking at sedimentary rocks of different ages, scientists can figure out how climate and environments have changed through time. Fossils of ancient living things are preserved in sedimentary rocks too! Sedimentary rocks are classified into 3 groups based on how they originated. Sandstone, shale and conglomerates are examples of clastic sedimentary rocks. These rocks form from sediments that were under pressure which caused water around the sediments to be squeezed out and the sediments become cemented together. Rock salt and gypsum are examples of chemical sedimentary rocks. These rocks formed when rocks dissolved in water and then the water evaporated and the minerals that were in the rocks crystallized into large deposits. Limestone and coals are examples of organic sedimentary rocks. These rocks formed from the sedimentary remains of shells, skeletons and other plant and animal parts. Taken from: http://www.geocities.com/RainForest/Canopy/1080/sedimentary_formation.htm OBSERVING HOW SEDIMENTARY ROCK FORMS Objectives: 1. Students will have a clear understanding how sedimentary rock forms. 2. Students will understand that sedimentary rock is made from other rocks by erosion. 3. Students will understand how water plays a big role in making sedimentary rock. Materials: Pebbles Water sand clear jar with lid bigger rocks spoon Procedure: 1. Place rocks and sand in glass jar and place lid on tightly. 2. Shake jar and observe. Have the rocks shifted? What rocks are on top? What rocks have dropped to the bottom? 3. Add water to jar so that the water covers all the rocks. 4. Shake and observe. Is there a change? Did the rocks move faster or slower in the water? Results: 1. How many seconds did you shake the jar with rocks until you saw a shift in where the rocks rested in the jar? No water___________seconds water_____________seconds 2. Did the sand stay on top or did it sink? Did you notice a difference when the water was added? __________________ __________________________________________________ __________________________________________________ 3. What form of weathering do you think shaking might mimic on Earth?_____________________________________________ Peanut Butter and Crackers Sedimentary Rock Problem: What is a delicious way we can demonstrate a sedimentary rock formation? Hypothesis (intelligent guess):__________________________ Materials: 2 crackers Jelly Chocolate chips Plastic knife crunchy peanut butter sliced bananas plate Procedure: 1. Lay one cracker on a plate 2. Use the knife to spread a layer of peanut butter on the cracker. 3. Add a layer of jelly on top. 4. Add a banana and a couple of chocolate chips. 5. Place the second cracker on top. 6. Eat the sedimentary rock sandwich! Results and Conclusion: Sedimentary rocks are formed from loose particles that have been carried from one place to another and redeposited. These sediments form layers similar to the layers in the sandwich. Each layer can be distinguished by differences in color, texture, and composition. The oldest layers are on the bottom and each new layer forms on the old one. The layer become compacted and cemented together over time to form solid rock. The chocolate chips show that sedimentary rock does not always have the same consistency throughout each layer. There could be fossils, shells, or other rocks in the layers. Lesson III Igneous Rock The oldest type of all rocks is the igneous rock. The word igneous comes from the Greek word for “fire.” Igneous rocks are formed from molten lava or magma. The hardening and crystallizing of magma formed igneous rock. Magma is hot liquid rock that stays inside the earth, but once this hot liquid reaches the Earth’s surface through a volcano, it is called lava. The different kinds of igneous rocks form depending on how fast the lava or magma cooled. Extrusive igneous rocks form when magma reaches the surface of the Earth’s or ocean’s floor. Extrusive igneous rocks cooled quickly creating rocks with small crystals. Basalt is the most common type of rock that is formed from lava. Basalt makes up most of the ocean floor. The crystals in basalt are so small that, even with a magnifying glass, they are hard to see. Obsidian is an example of extrusive igneous rock that has a shiny, glassy texture. It forms by very rapid cooling of lava. These rocks are very hard, and when they break they will have very sharp edges. Obsidian has been popular since the stone-age for making spear and arrow heads for cutting and hunting. Pumice is a very light porous igneous rock that formed during volcanic eruptions. The rock looks a little like foam that has hardened. In fact, it forms from frothy lava that is full of gas bubbles that cools quickly not giving the gas bubble time to escape. Pumice can be as a beauty aid to remove dead skin and calluses from feet and hands. Intrusive igneous rocks form under the earth’s surface. The hot magma cool underground and hardens into solid rock. Granite is an example of intrusive igneous rock. Granite is made up mostly from a mixture of quartz, mica, and feldspar crystals that can be seen very easily. http://www.geocities.com/RainForest/Canopy/1080/igneous_formation.htm Lab #3 Igneous Rock Question: How can I tell the difference between extrusive and intrusive igneous rock? Objectives: 1. Students will be able to distinguish the difference between intrusive and extrusive igneous rock and name 3 distinguishing features of each. 2. Students will understand the difference between lava and magma and explain how each is formed. 3. Students will name 2 igneous rocks and their consumer use. Materials: Pumice granite Obsidian basalt Magnifying lens worksheet Labeled pictures of different kinds of igneous rocks Procedure: 1. Set up 4 workstations with 3 different rocks at each station for a total of 12 rocks and 4-5 magnifying lens. 2. Students are to make observations of each rock sample and list characteristics of each. 3. Students are to infer and hypothesize the name of each igneous rock. 4. After all data is charted, play Igneous Tic-Tac-Toe. 5. Make a tic-tac-toe board on the white board. Divide the class into 2 teams. Decide how team captains will be picked. 6. The team captain must work with the rest of the team to come up with the answers, but only the team captain can give the answers and make the mark on the white board. Below are some ideas for questions. Questions for Tic-Tac-Toe 1. When magma comes out of a volcano it is called___? (lava) 2. Lava cools and forms____? (igneous rock) 3. The oldest type of rock is called___? (igneous rock) 4. Igneous rock that cools slowly is called__? (intrusive) 5. Lava that cools rapidly is called___? Extrusive rock) 6. Granite is a type of ____ igneous rock. (intrusive) 7. Obsidian is a type of ____ igneous rock (extrusive) 8. How do igneous rocks get large crystals? (they cool slowly) 9. Igneous come from the Greek word___? (fire) 10. This type of igneous rock has large pores. (pumice) 11. An igneous rock that can be found on countertops. (granite) 12. The black specs in granite are what type of crystal? (mica) 13. What is he most common type of rock formed from lava? (basalt) 14. This kind of rock was used to make spears and arrows. (obsidian) 15. This rock is used to get rid of calluses on feet. (pumice) NAME__________________________ Igneous Rock Student Worksheet TYPE OF IGNEOUS ROCK BASALT PUMICE GRANITE OBSIDIAN ROCK CHARACTERISTICS LESSON IV METAMORPHIC ROCK Pressure and heat can change many things, including rocks. The name for rocks that have undergone a change is called metamorphic rocks. Metamorphic comes from the Greek words meaning “change” and “form.” Metamorphic rocks form deep in the Earth where high temperature, great pressure and chemical reactions cause a sedimentary, igneous and even metamorphic rock to change into a new type of metamorphic rock. Metamorphic rocks are usually much harder than the original rocks and they often look like they have stripes. These banded or foliated areas are caused by different minerals in the rock that have been pressed into bands by heat and pressure. Metamorphic rocks can take a few million years to form. The intense heat comes from magma and the pressure comes from layers of rock piled on top of layers and layers of rock. The thicker the layers, the more pressure there is. These conditions can cause chemical changes on the rock as well as change the mineral structures in the rock. Some metamorphic rocks look like they have layers similar to what you see in sedimentary rocks. The minerals in the rock cause these layers to line up in the same direction when they are put under great pressure. Below are examples of metamorphic rocks Here are some examples of metamorphic rocks. Sandstone ------- Quartzite, Shale --------- Slate Limestone ------- Marble, Granite---------- Gneiss http://www.geocities.com/RainForest/Canopy/1080/metamorphic_formati on.htm Classroom Demonstration In order for students to understand how pressure can cause heat have them put their hands together and press hard. Their hands should get warmer. Have students put there hands together and rub them firmly. Are they getting warmer? Making Metamorphic Play Dough Objectives: 1. Students will observe how pressure can directly effect the position and alignment of mineral particles in metamorphic rock. 2. Students will observe how heat melts rock and minerals causing the texture to change. Materials: Gallon size Ziploc freezer bag 1c flour 1c salt warm water Hard straight candies like good n plenty Wax paper rolling pin Procedure: 1. Add 1 cup of salt to Ziploc bag. Have students feel the sandy, grainy texture. 2. Add 1 cup of flour. 3. Add ½ cup of warm water. Get all air out of the bag and zip it up. 4. Slowly and carefully knead the bag. Add more water if necessary until the mixture creates a round ball. Notice the texture of the dough. 5. Add a handful of candy to the mixture. Knead again until the candy pieces are mixed in. 6. Remove the play dough from the bag and divide it so each student has a portion of the dough. 7. Observe the positions of the candy pieces (up-down, back and forth). 8. Place the dough on a piece of wax paper. Add more flour if necessary. Using the rolling pin or wooden dowel, roll the dough into a thin pancake. What happened to the candy pieces? Lesson V The Rock Cycle We have learned that igneous, sedimentary and even metamorphic rock can change into a new kind of metamorphic rock. Rocks are constantly being formed, worn down and formed again. The process of rock changing is called the Rock Cycle. The rock cycle mean that rocks are recycled into something new. Some rocks have been around for more than 4 billion years. It can take millions of years for a rock to metamorphisize into a new rock. The journey of the rock cycle goes like this. In the mantle red hot lava is being pushed up towards the earth’s crust. Some of the magma creeps into the cracks of the volcano while the rest is forced out of the top of the volcano. The lava cools and forms igneous rocks. Igneous rock breaks down due to erosion and forms very small rocks or grains. These rocks form layers and are pressed and cemented together forming sedimentary rocks. Sedimentary rocks that are on the bottom build up pressure from the weight of the other rocks. They also become very hot due to the magma. This heat and pressure cause the sedimentary rock to change into metamorphic rock. When the metamorphic rock becomes deeply buried, after millions of years, it gets extremely hot and melts. Once again, it becomes magma and the cycle starts over again. Taken from: http://www.bbc.co.uk/education/rocks/rockcycle.shtml Key Factors to the Rock Cycle Erosion Erosion is the wearing away and transport of Earth’s materials. The major causes of erosion are water, wind and ice. Running water is said to be the greatest of all erosive actions. As running water moves over the hardest of rocks, it can in time wear them away. Water also is a great transportation device to move materials into ponds, lakes, rivers, oceans and streams. The continuous beating of ocean waves on land and the washing up of soil and rocks all contribute to the erosion of the seashore. That is why you often find sand and tiny pebbles on beaches. It also creates the smooth river and beach rocks that look polished. Wind carries away tiny particles of soil. Dust storms, created by large gusts of wind, show how sand, soil and small rocks are eroded away from the land. Pismo Beach is a great place to see how wind forms dunes of sand caused by the speed of wind. As windblown sand hit solid rock, the rock slowly wears away due to the abrasive action. Ice and snow move over land wearing and taking rocks, soil and other materials with them. These materials become deposited along the sides of glaciers forming u-shaped valleys. The freeze thaw cycle causes mountains to crumble over time and large rocks to break down to little rocks. When water gets into cracks of the rocks, this water expands during the winter, making the cracks larger. This annual cycle causes the cracks to get larger and larger until they eventually break causes smaller rocks to form. Class Demonstration I The Effects of Water Erosion Fill a glass bottle of water completely to the top and place the lid on firmly. Put the water bottle in a bag and place in the freezer. Carefully take the water bottle out of the freezer. What happened to the glass bottle? Why? Class Demonstration II Hot Rocks Objectives: 1. Students will see the effects of temperature change and weathering. 2. Students will understand the physical and chemical process that breaks down rock at the Earth’s surface. Materials: Candle Glass marbles Pliers Matches clear glass cold water gloves Procedure: 1. Put on the gloves as insulators because the heat will make the pliers very warm. 2. Have an adult light the candle with the matches. 3. Use the pliers to hold the marble over the candle for one minute. 4. Quickly place the marble in the glass of cold water. 5. Take out the marble and observe. Results and Conclusion: The marble cracked due to the extreme temperature difference. The same thing happens when rocks are heated by the sun and then suddenly cooled by rainwater. Weathering is the chemical and physical process that breaks down rock at the Earth’s surface. Lab Experiment Erosion Objectives: 1. Students will be able to identify 4 types of erosion; ice, wind, water and chemical. 2. Students will be able to determine what kind of erosion took place by looking at different landforms. Procedure: 1. Divide students into 4 groups. Explain to them that they will be able to experience all 4 stations set up in the classroom. 2. Discuss safety and laboratory protocol with students. 3. Handout student worksheets and explain they are to fill in the answers to each lab section immediately after experiencing the lab and before proceeding to the next table. 4. While they are waiting for other students to finish, students are to work on the word search provided. 5. Make a copy of the below procedures for each lab and place it at each station with the necessary materials to complete each experiment. 5 Lab Stations: (5 students per stations) Lab Station I- Wind Erosion – Sand Dunes Materials: sand, box with tall sides Procedure: Carefully, blow on the sand. Observe what happens. Record your results. Lab Station II – Water Erosion Materials: 1 cup measuring cup, water, soil Procedure: 1. Go outside where the soil is dry and bare. 2. Pour 1 cup of water slowly near the ground. 3. Now, pour 1 cup of water on the same spot, but this time hold the cup from as high of a distance as you can. 4. Observe the difference. Record your results. Lab Station III – Chemical Erosion Materials: chalk, plastic container, vinegar, eyedropper Procedure: 1. Place a piece of chalk in the plastic container. 2. On the student worksheet, hypothesize what will happen when the vinegar comes in contact with the chalk. 3. With the eyedropper, add 5 drops of vinegar slowly to the piece of chalk. 4. Record your results. Lab Station IV – Ice Erosion Materials: ice cube, modeling clay, sand, spoon, paper plate Procedure: 1. Take a piece of modeling clay and try to flatten it like a pancake. 2. Place the flattened piece of modeling clay on the paper plate. 3. Take an ice cube and rub it against the modeling clay a few times. Record your observations. 4. Place a spoonful of sand on the modeling clay. Put the ice cube on top of the sand and wait one minute. 5. After one minute, pick up the ice cube and look at the side that way lying on the sand. Record your observations. 6. Place ice cube back on the sandy modeling clay with the same side of the ice cube touching the sand. 7. Rub the ice cube back and forth across the surface. 8. The ice cube should be removed and the sand needs to be wiped off the clay. Record the clay’s surface texture. Erosion Worksheet Lab Station I- Wind Erosion 1. How do sand dunes form?_________________________ 2. Could you make the whole pile of sand move if you blew long enough?_____________________________________ Lab Station II-Water Erosion 1. How did the earth change when you poured your first cup of water?______________________________________ 2. How did the earth change when you poured the second cup of water from a higher elevation?________________ Lab Station III – Chemical Erosion 1. What happened when you dropped the vinegar onto the chalk?________________________________________ 2. What kind of reaction occurred? ____________________ Lab Station IV – Ice (Glacial) Erosion 1. What happened to the clay the first time you rubbed the ice cube on it?__________________________________ 2. What happened to the ice cube after it sat on the sand? 3. What happened to the clay when you rubbed it with the sand on it?_____________________________________ __________________________________________________ Name_______________________ Erosion S G U S S L F T G V N R S O I R H E V E C A E N O C I N G B E H M O D D O C I E S W N M T D J B N L L I S I O M E X I W L L I U O O O M H M O E H N E U W V G C R G P E U E X C E A O C I M E J R Y S N Q H G R T B S C X R O C K K U T J C A H T D E O M G G L A C I A L L E S N I A T N U O M P X R R M R G E T E M P E R A T U R E Y I S E L B B E P S M N A I T C N M A X X A M D S A H W O A W G V N A I B E G O X N F T W N U J E X C M U K W P F D Q P J O BOULDERS EROSION GLACIAL METAMORPHOSIS PEBBLES SEDIMENTARY WEATHERING CEMENT GEOLOGIST ICE MINERAL ROCK TEMPERATURE WIND CHEMICAL GEOLOGY IGNEOUS MOUNTAINS SAND WATER Lesson VI What is a Mineral A mineral is a solid material made from one substance that occurs naturally on Earth. A mineral may be made from one or more element that is uniformly distributed. Most of the common minerals are crystals. A crystal is a solid and commonly has a definite chemical composition and geometric shape. Quartz is a common crystal made from one substance SiO2 (silicon oxide). The quartz you find in Asia will have the same chemical composition as the quartz you find in California! There are three main types of minerals. Metallic minerals are copper, silver, mercury, nickel, gold and iron. Most metallic minerals are found in combination with other minerals, such as ores. Nonmetallic minerals are also very important to many industries. For example, graphite is used in pencils, halite is rock salt, borax is used in cleaning, talc is used in baby powders and sulfur is used as a preservative. The minerals that are found in mountains and valleys are called rock-forming minerals. These minerals are mainly silicates like quartz (SiO2). Silicon is a nonmetallic substance, always found in combination with something else. It is second, only to oxygen, as the number one element forming the Earth’s crust. There are many other rock-forming minerals such as: micas, feldspars, and garnets. There is a great difference in the way different minerals look. Minerals can be dull or sparkle in the light. Some minerals are so soft they can be scratched easily with your fingernail, while others are so hard they can scratch steel. There are many ways that scientists classify or group minerals. In this lesson we will learn about five properties. A property is a characteristic of a mineral. Properties help geologist better understand how the mineral was formed and also to help identify a mineral. The five properties that we are going to study today are luster, cleavage and fracture, hardness, color (streak), and magnetism. Property Definitions *Luster – Luster is the minerals ability to reflect light. Metallic minerals shine like metal and usually are full of luster. Some minerals have a glassy luster, such as Aragonite. Howlite, another mineral, often has a dull luster. *Cleavage or Fracture – Some minerals have a tendency to split or crack along parallel or in smooth lines. This property is easily seen in some minerals and can be tested by breaking it with a hammer. When a mineral makes a clean break it is called cleavage planes. Mica is an example of a mineral that splits easily and is said to have perfect cleavage. Fracture is related to cleavage and occurs when a mineral breaks at random lines or has rough edges. Obsidian and quartz are examples of minerals that have fracture. *Hardness- In 1822 a German scientist by the name of Frederick Mohs set up a scale from 1-10 to determine the approximate hardness of minerals. The scale is arranged from softest (Talc) to hardest (diamond). The scale is not made in exact proportion. In other words, diamonds are not 10 times harder than talc; Diamonds are actually 40 times harder! MOHS SCALE OF HARDNESS MINERAL TALC GYPSUM CALCITE FLUORITE APATITE FELDSPAR QUARTZ TOPAZ CORUNDUM DIAMOND HARDNESS 1 2 3 4 5 6 7 8 9 10 *Color – Color is the easiest property to see, but it is not always the best way to identify a mineral. Many minerals have more than one color due to the impurities that were present when the mineral was being formed. For example, quartz crystal can be found is clear, pearl, yellow, brown, pink, purple, blue and black. A better way to test the true color of a mineral is using a streak test. Streak is a test used by geologist to see the color of a mineral under the top layer of a mineral or coating on the mineral. Using a porcelain tile as a streak plate, rub the mineral across the tile. This is the minerals true color. The outer color of minerals can change when they are exposed to the atmosphere and gasses like oxygen. *Magnetism- This is probably the easiest property to test. Using a magnet, the students will touch the mineral to see if it is magnetic. Testing Minerals Objectives: 1. Students will be able to test, understand and define the following properties of minerals: hardness, color, magnetism, luster and cleavage. 2. Students will understand Mohs scale for hardness and be able to use everyday items to test minerals. 3. Students will understand the definitions of the following words as they pertain to geology: luster, hardness, property, streak, rock, mineral, cleavage, texture, color and geology. Materials: Worksheet pencil At least 5 different mineral varied in properties Non-glazed porcelain plate Magnet Penny steel nail Steel file flashlight Hammer goggles Procedure: Have five stations set up around the room. Each group of students should have 5 different minerals to test. Each mineral should be numbered from 1-5. They should take these minerals, as well as their worksheets to each station. Have the following materials set up at each station. Station I – Luster Material – Flashlight Procedure: 1. Using the flashlight, observe each mineral for its ability to reflect light. 2. Number the minerals in order of least “shiny” (1) to most “shiny” (5). 3. Record the results. Station II – Cleavage or Fracture Materials – small hammer goggles Procedure: 1. Students need to wear goggles to protect their eyes and need to be cautioned with using tools. 2. It may be a good idea to have adult supervision. Some students may get carried away! 3. Students are to lightly tap each mineral with the hammer. 4. Record the results. Station III – Hardness Materials – penny fingernail (on your hand!) Iron nail steel file (fingernail file) Mohs Hardness scale Procedure: 1. Students will test the hardness of all five minerals in this order: (1) fingernail (hardness 2.5), (2) penny (hardness 3.0), steel nail (hardness 5.5), and steel file (hardness 7.0). 2. If the finger nail can scratch the mineral, the mineral has a hardness of 2.5 or less. There is no need to continue testing. Continue testing each mineral until you can scratch it with one of the above materials. If you are unable to scratch the material after using the file, record your observation as a 7+ because it is harder than you are able to test. 3. Record your test results. Station IV – Color or streak test Materials: porcelain non-glazed tile Procedure: 1. Taking one mineral at a time, rub it against the tile until a color is observed. 2. Record your results. Station V – Magnetism Materials: strong magnet or compass Procedure: 1. Move the magnet or compass over the mineral. 2. Observe and record your results Properties of Minerals – Worksheet 1. How can you tell if a mineral has Luster?____________ 2. Record the degree of luster from 1-5 (one having very little, 5 having a lot of luster). Mineral number 1 2 3 4 5 Degree of Luster 3. What is the difference between cleavage and fracture? 4. Name the mineral(s) that have cleavage______________ Name the mineral(s) that have fracture_______________ 5. Record your observation when testing mineral hardness. Mineral Number Degree of hardness 1 2 3 4 5 6. Why is a streak test a good way in determining a minerals “true” color? 7. Record the color observed of each mineral tested. Mineral Number Tested 1 Color Observed 2 3 4 5 8. Record the magnetism property of each mineral. Mineral Number Tested Magnetism Observed (Y or N) 9. Why do you think it is important to test the properties of minerals?______________________________________ Rock and Mineral Vocabulary Across 3. A scientist who studies rocks 4. A crack or break along flat surface 5. Means comes from fire 7. How a rock or mineral feels 9. A solid element or compound in nature 10. A character trait of a mineral 12. Soft layered rock, may have fossils 13. Ability of mineral to be scratched Down 1. The way minerals reflect light 2. Changed rock from heat & pressure 6. Color on porcelain tile 8. The study of the earth 11. The number of major rock groups Lesson VII Mystery Mineral Objective: Students will be able to identify a “mystery” mineral by testing its properties and using research materials. Purpose: 1. Students will test mineral properties to determine what mineral they have. 2. Students will work in small groups and test minerals for the following properties: color, streak, texture, hardness, luster, cleavage, fracture, weight and magnetism. 3. Students will form a hypothesis as to what their mineral is before beginning the experiment. 4. Students will research their mineral and give 3 facts they learned about their mineral. Materials: Lab stations set up as in Lab #6 with supplies listed Books on minerals internet Worksheet colored pencils Magnifying lens Name_______________________ Mystery Mineral *Making an intelligent guess, name your mineral. Hypothesis: I think my mineral is_______________________ Draw a picture of your mineral Using colored pencils Draw a close up of your mineral using the Magnifying glass Properties of Mystery Mineral 1. The color of my mineral is________________________ 2. Using the streak test, my mineral color is____________ 3. Hardness a. can the mineral be scratched with fingernail?___ b. scratched with a penny?______________ c. scratched with steel nail?_____________ 4. My mineral feels (soft, rough, bumpy, smooth)________ 5. Is your mineral attracted to a magnet?_______________ 6. Does you mineral have luster?_________If so, how much? ____________________________________ 7. Does your mineral have cleavage or fracture? _____________________________________________ My Mystery Mineral is_________________________ Find out 3 interesting facts about your mineral. 1.________________________________________________ 2._________________________________________________ 3._________________________________________________ Lab #VIII Minerals for Health Every year 46,414 pounds of new minerals must be provided for every person in the United States to make the things we use for everyday use according to the Mineral Information Institute. Minerals are required to provide the basic needs of food, clothing and shelter. We need minerals to drive a car, use a computer, and speak on a telephone and even cook. The average person doesn’t go to the grocery store with minerals on their grocery list, yet nearly everything we do and everything we use, requires the mining somewhere to produce the natural resources that makes the things we use. All living things need the fuel provided by minerals and metals. Life processes cannot occur without minerals. There are 14 needed minerals necessary for healthy plant growth and many more important minerals necessary for good health in humans. Minerals are so important for proper nutrition that they are often added to vitamin pills. Next time you take a vitamin, look at the label and it will list important vitamins as well as minerals for good health. Zinc, a common mineral, has been sold as lozenges to help with cold symptoms. Iron is an important mineral given to people with anemia (low red blood cells), because of its ability to increase red blood cells in the body. Calcium is important for healthy bones and teeth. Many foods are fortified with vitamins and minerals to make them healthier to eat. When a food is fortified it means that vitamins and minerals are added to give the food a higher nutritive value. Breakfast cereal is a great example of a food product that has been fortified with vitamins and minerals. After the cereal is made, vitamins and minerals are actually sprayed onto the cereal. This is why it is important to drink the milk at the bottom of you cereal bowl because some of the minerals may wash off in the milk! Even though minerals are important part of good health, it is wise to follow the guidelines your doctor gives you as to how much you should have. If your body has too much or too little of a certain mineral, it can make your body sick. There is Iron in My Cereal! Overview: Many cereals are fortified with iron as well as other minerals and vitamins. The iron used in cereals is a metallic form that breaks down in the stomach and is absorbed in the intestines. Iron is an essential part of our diet. Iron is necessary in making hemoglobin, the compound that makes red blood cells red in color. If your body has too little iron you can become very tired and have a higher chance of getting sick. You can also have too much iron in your body so it is important not to have more than what your doctor recommends. FUN FACT – Our body only has enough iron to make 2 small nails! Objective: 1. Students will be able to separate the iron from the iron fortified cereal using a magnet. 2. Students will understand the importance of minerals in maintaining good health. Materials: Good strong magnet Water Plastic stir stick 1 quart size Ziploc bag Fortified with iron cereal a small bowl clear plastic cup hand lens Procedure: 1. Examine a piece of cereal closely. You probably won’t be able to see the iron, but it is there. 2. Place a few flakes of cereal in the plastic bowl. Hold the magnet over the cereal to see if they are attracted or repelled by the magnetic field. There is a good chance that the cereal doesn’t respond to the magnet. This is due to the friction between the flakes of cereal. 3. Add water to the flakes of cereal in the bowl. Now hold the magnet close to the flakes and see if you can move them. Any movement that occurs will be slight, so be patient! With practice you might even be able to rotate the flakes in a circle with the magnet. The water reduced the friction between the flakes of cereal. 4. Fill your zip-lock bag half full of cereal. Seal the bag slowly trying to remove any air from getting trapped in the bag. Crush the cereal as finely as you can by squishing the bag. Be careful not to pop the bag or put a hole in it. This is similar to the process used by miners when they crush rock from their mine in order to release the iron from it. 5. Pour enough water into the bag to create a thin cereal paste. It should have the consistency of a thick soup. 6. Pour your soupy cereal into a clear plastic cup. 7. Hold the magnet against the outside of the cup while you stir the mixture gently with a plastic stirrer. This will cause the microscopic iron particles to pass through the magnetic field of your magnet. The tiny black particles are freed during the crushing process and will begin to accumulate at the side of the cup where the magnet is. The concentration of iron will build over 2-3 minutes and you should be able to see the iron. Use a hand lens to see the particles better. Can you see them? Iron Ore http://www.mii.org/Minerals/photoiron.html Lesson IX Volcanoes Beneath the crust of the earth is a large mass of molten, liquid rock called magma. The magma is very hot creating tremendous pressure. When a break or weak spot occurs within the Earth’s crust, the pressure is released sending magma to the Earth’s surface. When magma reaches the Earth’s surface it is called lava. As the pressure builds in the mantle, the earth’s crust raises creating mountains or hills. The opening at the top of a volcano is called a crater. During an eruption, the crater releases gases, rocks, lava, cinder, ashes, loud explosions ad rumbling. A volcano has one large vent in the middle, where the crater forms and it has smaller vents coming out from the larger vent. Igneous rock layers are formed by the magma that has been forced into the vents. If the igneous rock is forced into cracks and crevices below the surface of the volcano it is called sill. When igneous rock is forced into the volcano, but doesn’t break through the earth’s crust it is called a dike. Some volcanic eruptions are explosive and others are not. How explosive a volcano is depends on the magma. When magma is thin and runny, gasses can escape easily making the magma flow in a nonviolent way. If the magma is thick and sticky, gasses cannot escape easily. Pressure builds up until the gasses escape violently and explode. http://interactive2.usgs.gov/learningweb/images/volcanoes_intro01.jpg Making a Model Volcano Objective: 1. Students will be able to make a model volcano and recognize and identify the following parts: crater, vent, magma, and lava. 2. Student will understand how volcanoes act as cooling vents from the hot magma in the mantle layer. Materials: Salt dough Small plastic water bottle Vinegar Baking soda Red food coloring (optional) cardboard box liquid detergent funnel newspaper warm water Procedure: 1. Place an empty soda bottle in the middle of a cardboard box. Take salt dough and shape it around the outside of the bottle being careful not to cover the hole on top or get anything inside the bottle. 2. Shape the dough to make it look like a volcano. 3. Fill the water bottle ½ way full with warm water. 4. Add 5 drops of liquid detergent. 5. Add 2 drops of red food coloring. 6. Using a funnel add 2T baking soda. 7. Add ¼ cup of vinegar slowly using a funnel. Remove the funnel and observe. Results: You should see red foamy “lava” oozing out of the model volcano. The bubbles are formed from a chemical reaction between the baking soda and vinegar releasing a gas, carbon dioxide (CO2). This gas is the same as the gas released from a volcano was the magma is expelled from the mantle. A volcanic eruption occurs when the magma overflows out of the earth’s crust where lava is formed. Lesson X Earthquakes! Earthquakes are the shaking, rolling and movement of the Earth’s surface. Earthquakes are caused when the stress within the crust of the Earth builds up, causing an area of rock to move (release pressure) along a fault. A fault is a fracture within the earth’s crust caused by vertical slipping or folding. The point on the fault where the movement first begins is called the focus of the earthquake. The point on the surface of the Earth directly above the focus is called the epicenter. When an earthquake occurs, energy is released. This energy is released in the form of seismic waves. These waves travel throughout the earth and transfer energy from one point to another. The energy released during an earthquake causes the ground to shake. If you are close to the epicenter of an earthquake, the shaking is more severe than if you were farther away. Earthquakes have two main kinds of waves of vibration. The Primary waves are waves that are often heard before they are felt and Secondary waves are waves that twist and shake the ground and cause most of the damage. There are many ways to measure the intensity of an earthquake. One way is called the Modified Mercalli. This method is measured by how people react and feel towards the earthquake. A more recognized and reliable method is called the Richter Scale. The Richter scale is a mathematical measurement of the intensity of the ground shaking, as measured on a seismograph. It is actually a measurement of the size of waves produced by the earthquake. Earthquakes below 4.0 on the Richter scale usually do not cause damage. Earthquakes over 5.0 on the scale can cause damage. A magnitude 6.0 earthquake is considered strong and a magnitude over 7.0 is a major earthquake. The most important thing to remember during an earthquake is safety. It is important to get under a table or desk and cover your head by putting your hands behind your neck, face down. If the shaking gets violent, try to hold on to the legs of a table or desk and keep under it. If you are outside, stay away from buildings. Interesting facts *The San Andreas Fault is over 800 miles lon! After shocks are small earthquakes that occur after major earthquakes. Class Demonstration By using a slinky you can visually demonstrate the difference between P-waves and S-waves to you students. With two student volunteers holding the slinky in an outstretched parallel position, have one student pull slightly on the slinky. Then have the other student pull slightly on the slinky. Observe the slinky move in and out. This is a great example of a P-wave. To show an example of a S-wave have students sit in the same position, but instead of moving the slinky in an up down path, have the students move the slinky from side to side. The slinky will look like it is slithering like a snake. This is similar to how S-waves move through the Earth. http://regentsprep.org/Regents/earthsci/units/earthquakes/earthquakes.cfm Class Demonstration Gelatin Earth Purpose: It is hard to understand how the Earth moves and how it can damage buildings and other materials. This lesson will help students visually understand what goes on during an earthquake. Materials: Prepare 4/ 6oz. boxes of gelatin dessert and place in a 9X12 metal baking pan. sugar cubes Plastic wrap plates spoons Procedure: 1. Show students the cooled gelatin and tell them this represents the Earth. 2. Gently tap the sides of the gelatin pan. You can see waves traveling through the gelatin. This is what an earthquake wave looks like when it goes through the earth. Tap the pan harder and see how the waves get bigger. 3. Cover the gelatin with plastic wrap. Make sure the plastic wrap is touching the gelatin. 4. Using sugar cubes, build a 2X2 house on top of the gelatin. Tap the pan lightly. What happened? 5. Tap the pan harder. What happened? 6. Now, build a 2X2 house and a 3X3 house on the gelatin. Tap lightly. Observe. Tap harder. What sugar cube house fell first? Why? If time permits divide the students small groups. Using a spatula, slice the gelatin into cubes and give each group a square of gelatin. Have the students try to “build” their own sugar cube houses. Simulate earthquakes. Use frosting as glue to hold the sugar cubes together. Have fun! Useful Internet Sites http://library.thinkquest.org/J002289/. This Planet really Rocks; All about Rocks and minerals. A great site with fun facts. It’s written so students can easily understand. www.mii.org. Mineral Information Institute. An excellent site about mineral with lots of free lesson, worksheets and valuable information about minerals. http://iknowthat.school.aol.com/AOLAtSchool/volcano/volcano_movie. html. A wonderful interactive volcano to watch. Students can observe what really happens when a volcano is active with this illustrated animation. http://www.nationalgeographic.com/ngkids/0403/main.html. Information on the 1994 Northridge Earthquake and other earthquakes. http://www.nationalgeographic.com/ngkids/0312/main.html. Excellent presentation on volcanoes. http://www.nationalgeographic.com/forcesofnature/interactive/index.h tml?section=e. This site has a level where you can change the intensity of an earthquake demonstrating what happens at different intensities. http://www.minsocam.org/MSA/K12/uses/uses.html. A fun game like activity where students can find out what minerals they have in their house and how they are used. http://earthquake.usgs.gov/4teachers/EQ101.htm. A terrific power point presentation on earthquakes. Geared for middle schoolers to high schoolers. http://library.thinkquest.org/J002289/rcycleact.html. A fun rock cycle activity. http://library.thinkquest.org/J002289/name.html. A fun interactive quiz on rocks and minerals. http://www.fi.edu/fellows/payton/rocks/index2.html. Rock Hounds. Just about everything to help you learn and teach about rocks and minerals. http://www.teach-nology.com/teachers/lesson_plans/. Great lesson ideas on geology. http://teachnet-lab.org/ps101/bglasgold/rocks/overview.htm. Let’s Rock! A unit on rocks and minerals for 3rd – 5th grade. http://school.discovery.com/lessonplans/. Discovery Schools has wonderful lesson plans on many subjects. http://www.eduref.org/cgi-bin/lessons.cgi/Science. Excellent lesson plans in many areas. http://www.fema.gov/kids/quake.htm. A great site for kids on earthquakes. http://www.data.scec.org/. The Southern California Earthquake Data center. http://www.msnucleus.org/membership/html/k6/pt/dictionary/ptdictionary.html. A great dictionary on volcanic and earthquake terms. http://www.cde.ca.gov/ta/tg/sr/documents/mineral5.pdf. mineral identification table http://www.stf.sk.ca/teaching_res/library/teach_mat_centre/tmc/e106 25/e10625.htm. A Rocks, minerals and fossils lesson for 4th grade. http://www.puzzlemaker.com/. A great site to make puzzles for your classroom.