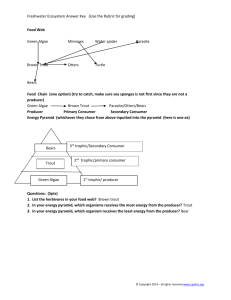
INTRODUCTION - USDA Forest Service
advertisement

MOLECULAR GENETIC INVESTIGATION OF YELLOWSTONE CUTTHROAT TROUT AND FINESPOTTED SNAKE RIVER CUTTHROAT TROUT A REPORT IN PARTIAL FULFILLMENT OF: AGREEMENT # 165/04 STATE OF WYOMING WYOMING GAME AND FISH COMMISSION: GRANT AGREEMENT PREPARED BY: MARK A. NOVAK AND JEFFREY L. KERSHNER USDA FOREST SERVICE AQUATIC, WATERSHED AND EARTH RESOURCES DEPARTMENT UTAH STATE UNIVERSITY AND KAREN E. MOCK FOREST, RANGE AND WILDLIFE RESOURCES DEPARTMENT UTAH STATE UNIVERSITY TABLE OF CONTENTS TABLE OF CONTENTS _________________________________________________ ii LIST OF TABLES ______________________________________________________iv LIST OF FIGURES _____________________________________________________vi ABSTRACT _________________________________________________________ viii EXECUTIVE SUMMARY ________________________________________________ix INTRODUCTION ______________________________________________________ 1 Yellowstone Cutthroat Trout Phylogeography and Systematics _________________ 2 Cutthroat Trout Distribution in the Snake River Headwaters ___________________ 6 Study Area Description ________________________________________________ 6 Scale of Analysis and Geographic Sub-sampling ____________________________ 8 METHODS ___________________________________________________________ 9 Sample Collection ____________________________________________________ 9 Stream Sample Intervals ____________________________________________ 10 Stream Sampling Protocols __________________________________________ 10 Fish Species Identification __________________________________________ 10 Fish Metrics, Photographs, and Tissue Samples _________________________ 13 Genetic Analysis ____________________________________________________ 13 Extraction of DNA _________________________________________________ 13 Methods by Objective ______________________________________________ 14 Objective 1 – Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions _____________________________________ 14 Objective 2a – Determine morphological differences between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape ________ 18 Objective 2b – Determine genetic differentiation between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape _____________________ 19 Objective 3 – Describe patterns of genetic variation in cutthroat trout within and among major drainages in the study landscape ________________________ 19 Objective 4 – Assess introgression with rainbow trout using both morphologic and genetic tools ________________________________________________ 20 Results _____________________________________________________________ 20 Survey Results _____________________________________________________ 20 Genetic Structuring __________________________________________________ 22 Results by Study Objective __________________________________________ 22 Objective 1 – Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions _____________________________________ 22 Objective 2b – Determine genetic differentiation between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape _____________________ 26 Objective 3 – Describe patterns of genetic variation in cutthroat trout within and among major drainages in the study landscape ________________________ 36 Objective 4 – Detection of Rainbow Trout Introgression __________________ 41 Discussion __________________________________________________________ 44 Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions ____________________________________________ 44 ii Genetic Differentiation among Morphotypes _________________________ 45 Genetic Differentiation among Major Drainages ______________________ 46 Detection of Rainbow Trout Introgression ___________________________ 47 Management Recommendations __________________________________ 48 References Cited _____________________________________________________ 49 APPENDIX A ________________________________________________________ 55 APPENDIX B ________________________________________________________ 64 iii LIST OF TABLES Table 1 Common and scientific names1 of fishes and amphibians in the Snake Headwaters basin of Wyoming, and species abbreviations as identified by the Wyoming Game and Fish Department. _________________________________ 12 Table 2 Polymerase chain reaction (PCR) primers used to amplify and sequence the ND1-2 region in cutthroat trout. Unpublished primer sources are noted: IDFG = Idaho Fish & Game Eagle Fish Health Lab; USU = Utah State University. ______ 15 Table 3 Sample subset used to assess landscape-scale sequence variation in the mitochondrial ND1-2 region and to design internal primers to capture this variation. _______________________________________________________________ 16 Table 4 Polymerase chain reaction (PCR) primers used to amplify and assess polymorphism at nDNA microsatellite loci in cutthroat trout. Unpublished primer sources are noted by place of origin: GIS = Genetic Identification Services. ____ 17 Table 5 Summary by river drainage for numbers of streams and stream reaches, and stream length (km) surveyed for cutthroat trout presence/absence between 1998 and 2003 in the Snake River headwaters of northwest Wyoming. River drainages are listed as they flow into the Snake River proceeding upstream from Palisades Reservoir. _______________________________________________________ 21 Table 6 Number of streams with cutthroat, brook, and rainbow trout present and the stream length (km) occupied, based on presence/absence surveys between 1998 and 2003 in the Snake River headwaters, Wyoming. ______________________ 21 Table 7 Presence of Yellowstone cutthroat trout (large spotted morphotype) and Snake River cutthroat trout (fine spotted morphotype) in streams surveyed, and stream length (km) occupied in the Snake River headwaters, Wyoming. _____________ 22 Table 8 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the upper Snake River drainage, Wyoming. Samples were pooled across all drainages. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). _______________________________ 26 Table 9 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Jackson Hole segment of the Snake River, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). _______________________________________________________ 26 Table 10 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Gros Ventre River drainage, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). ______________ 27 Table 11 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Hoback River, Wyoming. Distances within and between groups are expressed as iv average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). _______________________ 27 Table 12 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Snake River Canyon segment of the Snake River, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). _______________________________________________________ 27 Table 13 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Greys River, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). _______________________ 28 Table 14 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal, shaded) and between geographic groups of cutthroat trout in the Snake River headwaters, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). ______ 36 Table 15 Genetic diversity indices for cutthroat trout in Snake River headwaters drainages. Nucleotide diversity (), haplotype diversity (Hd), and number of haplotypes are presented for each drainage. ____________________________ 37 Table 16 Genetic differentiation among cutthroat trout in Snake River headwaters drainages, based on haplotype distributions, characterized using the GST statistic (Nei 1987; Hudson et al. 1992). ______________________________________ 37 Table 17 Locations and fish metrics for five rainbow trout (RBT) and seven rainbowcutthroat trout hybrids (RXC) captured in the Snake River headwaters, Wyoming. 42 Table 18 Summary of the number of records, by river drainage, of individual fish, and the approximate number of those fish that were photographed and/or a caudal fin clip collected. River drainages are listed as they generally occur from north to south. __________________________________________________________ 55 Table 19 Total genomic DNA extractions for several streams1 in each of five geographic areas. The number of extractions per stream varied due to stream length, numbers of fish captured over the minimum size (>150 mm), and number of samples available for each putative cutthroat trout morphotype1. The geographic areas are arranged as they generally occur from north to south. A history of cutthroat trout stocking in each stream is provided. ___________________________________ 56 Table 20 Lists the streams and samples, by geographic area1, selected for sequencing the mtDNA ND2 gene region. Contiguous sequences (~1,100 bp) of n=324 samples were completed. ___________________________________________ 59 Table 21 Number of cutthroat trout1 of the haplotypes A-M, per stream, within five geographic areas in the Snake River study area. The Snake River is split into two geographic areas, Jackson Hole and Snake River Canyon. The geographic areas are arranged as they generally occur from north to south. __________________ 61 v LIST OF FIGURES Figure 1 Historical transcontinental range of Yellowstone cutthroat trout, with finespotted Snake River cutthroat trout historical range indicated. ______________________ 3 Figure 2 Yellowstone cutthroat trout Oncorhynchus clarki bouvieri (YSC). This fish exhibits the YSC spotting pattern, with larger spots that are concentrated towards the caudal peduncle. ________________________________________________ 5 Figure 3 The finespotted Snake River cutthroat trout Oncorhynchus clarki subspecies (SRC) remains taxonomically undescribed. This fish shows the classic SRC pattern with small well distributed spots. _______________________________________ 5 Figure 4 A cutthroat trout exhibiting a common intermediate spot pattern, with small to medium spots that are concentrated toward the caudal peduncle. _____________ 5 Figure 5 Snake River headwaters study area, in northwest Wyoming (approximately 9,440 km2). The five geographic areas between Palisades Reservoir and Jackson Lake are: Jackson Hole, Gros Ventre, Hoback, Snake River Canyon, and Greys. _ 7 Figure 6 Occurrence of cutthroat trout morphotypes within mitochondrial haplotypes AM. YSC is a single occurrence haplotype from Yellowstone Lake; BRC is Bonneville cutthroat trout. Haplotype network was produced using statistical parsimony. ______________________________________________________ 29 Figure 7 Frequencies of three morphotypes and thirteen haplotypes in the Snake River headwaters study landscape. Occurrence of morphotypes was similar throughout each of the five geographic areas, whereas four haplotypes were dominant. ____ 30 Figure 8 Displays locations of streams in Jackson Hole from which samples were selected. Frequencies of the three morphotypes are on the upper left. Haplotype frequencies by specific morphotype are on the right. ______________________ 31 Figure 9 Displays locations of streams in the Gros Ventre River drainage from which samples were selected. Frequencies of the three morphotypes are on the upper right. Haplotype frequencies by specific morphotype are on the left. __________ 32 Figure 10 Displays locations of streams in the Hoback River drainage from which samples were selected. Frequencies of the three morphotypes are on the lower left. Haplotype frequencies by specific morphotype are on the right. ______________ 33 Figure 11 Displays locations of streams in the Snake River Canyon from which samples were selected. Frequencies of the three morphotypes are on the left. Haplotype frequencies by specific morphotype are on the right. ______________________ 34 Figure 12 Displays locations of streams in the Greys River drainage from which samples were selected. Frequencies of the three morphotypes are on the lower left. Haplotype frequencies by specific morphotype are on the right. __________ 35 Figure 13 Frequencies of haplotypes A-M varied among the five geographic areas within the Snake River headwaters, Wyoming. Four haplotypes were dominant, with two (B and D) occurring throughout the study landscape. Haplotype A was present mainly in the Hoback, Snake River Canyon and Greys. Haplotype C occurs mainly in tributaries in the Teton Mountains within Jackson Hole. _______ 38 Figure 14 Dendrogram of haplotypes A-M identified in the Snake River headwaters, Wyoming. Cutthroat trout out groups include: BRC – Bonneville, CRC – Colorado River, GBC – greenback, LHC – Lahontan, SRC02 – finespotted Snake River, vi WSC – west slope, YSCA1, YSCT1, YSC53 and YSC55 – Yellowstone. Rainbow trout (RBT) and rainbow-cutthroat hybrid (RXC) haplotypes are included in this unrooted neighbor-joining tree. Values at branches are the relative strengths of nodes (percent) assessed by bootstrapping 1,000 times; scale is 5 base pair difference. _______________________________________________________ 39 Figure 15 Network of cutthroat trout haplotypes A-M, with frequency of occurrence for each of five geographic areas indicated by symbols. YSC is a single occurrence haplotype from Yellowstone Lake; BRC is Bonneville cutthroat trout. Haplotype network was produced using statistical parsimony.________________________ 40 Figure 16 Presence of rainbow trout or rainbow-cutthroat trout hybrids in Gros Ventre and Greys River drainages were previously known or suspected. The capture of rainbow-cutthroat trout hybrids in the Hoback R, and upstream of Lower Slide Lake in the Gros Ventre were the first documented. ___________________________ 43 vii ABSTRACT We used a landscape scale approach to facilitate the synthesis of geomorphic, ecological, and genetic information regarding the distribution and organization of Yellowstone cutthroat trout, Oncorhynchus clarki bouvieri, and finespotted Snake River cutthroat trout, Oncorhynchus clarki subspecies, in the Snake River headwaters of northwest Wyoming. Selection criteria allowed us to hierarchically analyze for morphological or geographic structuring from the basin scale, to the stream reach scale. While we were unable to differentiate two distinct morphotypes, the conservation of unique color, spotting patterns, and life histories may be important for future management. Genetic differences among drainages were apparent, as evidenced by a) average pairwise nucleotide differences within and between drainages, b) a nonrandom distribution of haplotypes among drainages (2 = 232.67; P < 0.00001), and c) an overall pairwise GST of 0.14. Two distinct haplotype clades were present in the dataset. Clade 1 haplotypes tended to be more common in Jackson Hole and the Gros Ventre, and clade 2 haplotypes were more common in the Hoback, Snake River Canyon, and Greys. Morphological and genetic differences were observed in rainbowcutthroat hybrids that distinguished them from cutthroat trout. Hybridization was limited to those locales previously suspected of harboring RBT or RXC. Further work is recommended using the existing samples and markers developed from this effort, combined with additional collections from the Snake River. viii EXECUTIVE SUMMARY To date there have been no concerted efforts to determine whether Yellowstone cutthroat trout, Oncorhynchus clarki bouvieri (YSC), and finespotted Snake River cutthroat trout, Oncorhynchus clarki subspecies (SRC), in the Snake River headwaters differ with respect to ecology, morphology, and/or genetic characteristics. The majority of the research within the Snake River headwaters has described the biology and ecology of the SRC fishery in the mainstem Snake River in Jackson Hole (Hayden 1967; Wiley 1969; Hagenbuck 1970; Kiefling 1972, 1978; Harper and Farag 2002; Harper and Farag 2004). Previous genetic investigations of fish from both the mainstem and headwater tributaries were used to describe the phylogeography of the interior cutthroat trout (Murphy 1974), examine genetic differences between YSC and SRC (Loudenslager 1978), and used to compile a biological classification of native trout of western North America (Behnke 1992). Concern over the status of these fish prompted a status review of YSC following a 1998 petition to list them as a threatened species under the Endangered Species Act (WGFD 1999). The YSC was eventually found to be “not warranted” for listing, but the US Fish and Wildlife Service is under court order to re-examine the data used for the initial finding and proceed with a 1-year status review. The Snake River above Palisades dam has been identified as a large, relatively intact basin that represents one of the last strongholds of YSC and the potentially unique SRC morphotype. However, the status of cutthroat trout populations and the threats to these populations have never been formally investigated. This work examines three major questions that need to be answered in order to determine the status of cutthroat trout in the Snake River above Palisades dam. First, are there morphometric and genetic differences between YSC and SRC that would indicate that these are unique subspecies and that these fish should be managed separately? Second, if there are no apparent subspecies differences, are there differences in the genetic structure among geographic units that would indicate separate management units within the basin? Third, given the stocking history of rainbow trout and other nonnative cutthroat trout, where have populations within the basin been compromised by hybridization and where are the potential threats? We used these questions to develop the following study objectives: 1) Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions. 2) Determine morphometric and genetic differentiation between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape. 3) Describe patterns of genetic variation in cutthroat trout within and among major drainages in the study landscape. 4) Assess introgression with rainbow trout using both morphologic and genetic tools. No definitive description of the historical range of cutthroat trout in the Snake River headwaters exists. Behnke (1992) hypothesizes that only YSC were historically present in tributaries of the Snake River upstream of the Gros Ventre River confluence ix and in headwater streams of the Gros Ventre River drainage. He speculates that the finespotted morphotype historically occupied the Snake River downstream of Jackson Lake and all tributaries downstream of the Gros Ventre River. However, our distribution surveys suggest a pattern of headwater occupancy by YSC persists in each of the river drainages, as well as in many of the smaller tributaries to the Snake River. Present occupancy of cutthroat trout is >95% of streams, and >90% of stream length inhabited by trout. Cutthroat trout were found in 294 streams in 1,483 km of habitat. Nine rainbow-cutthroat hybrids were found in the Greys (2), Hoback (1), and Gros Ventre (6) areas during the survey. Brook trout were found in all areas. Yellowstone cutthroat trout (large spotted morphotype) were found in considerably fewer streams (102 streams, 277km) and in fewer locations than Snake River cutthroat trout (fine spotted morphotype; 258 streams, 1,249 km). The large and fine spotted forms were sympatric in 98 streams, representing 225 km. The SRC and YSC morphotypes are closely related (Loudenslager and Kitchin 1979; Loudenslager and Gall 1980), and F1 progeny exhibiting intermediate spotting patterns have been observed when hatchery stocks were combined with wild populations (Behnke 1992). Montgomery (1995) proposed to name the SRC subspecies Oncorhynchus clarki behnkei. However, the name was invalidated due to omission of a type specimen (D. Shiozawa, personal communication). The actual recognition of the SRC as a subspecies distinct from the YSC remains unresolved (Behnke 2002). Prior to our work, no information on genetic status of these fishes (e.g., introgression by rainbow trout) was available for our study streams. While non-native trout introductions have been widespread throughout the basin, displacement of cutthroat trout (e.g., by brook trout), and presumably introgression (e.g., with rainbow trout) are assumed to have occurred on a limited basis. Our landscape scale approach facilitated the synthesis of geomorphic, ecological, and genetic information regarding the distribution and organization of cutthroat trout within the river drainage, watershed, stream, or stream reach. Samples were selected for inclusion in this analysis based on trout external morphology (spot patterns). Streams were selected to be representative of five geographic areas comprised of four river drainages in the riverscape. Selection criteria allowed us to hierarchically analyze for morphological or geographic structuring from the basin scale, to the stream reach scale. Polymerase chain reaction (PCR) primers were developed for use with mitochondrial DNA in upper Snake River cutthroat trout. These primers reliably amplify a ~1,100 bp region of the ND2 mitochondrial gene, and can be used both for amplification and sequencing. Six polymorphic microsatellite loci were identified for use in Snake River headwaters cutthroat trout genetic analyses. The primer pairs reliably amplified these nuclear loci, and allele sizes ranged from approximately 100 to 400 bp. Multiplexing (simultaneous amplification) of several loci was not attempted pending determination of polymorphism for several primer sets. Genetic differentiation among morphotypes was not apparent, either within drainages or pooling across the entire study area. Differences in haplotypic composition among groups were likely due to sample size differences or stochastic sampling error. While we were unable to differentiate two distinct morphotypes, the conservation of unique color, spotting patterns, or life histories may be important for x future management. Unique phenotypes and life histories in westslope cutthroat trout, and physiological adaptations in related interior cutthroat trout are examples of such variation that exists and that should be maintained (Carl & Stelfox 1989; Taylor et al. 2003). Genetic differences among drainages were apparent in all analyses, as evidenced by a) average pairwise nucleotide differences within and between drainages, b) a non-random distribution of haplotypes among drainages (2 = 232.67; P < 0.00001), and c) an overall pairwise GST of 0.14. Two distinct haplotype clades were present in the dataset. Clade 1 was distributed throughout the study area, but there was a tendency for this group of haplotypes to be more common in Jackson Hole and the Gros Ventre, while clade 2 haplotypes were more common in the Hoback, Snake River Canyon, and Greys. These clades are likely to have evolved in response to different hydrogeographic conditions than those that exist today. The main identifying feature for all of the rainbow-cutthroat hybrid fish was white margins or tips on the pelvic and anal fins. Genetic differences were observed in 6 of the 8 hybrid fish that distinguished them from cutthroat trout. The two remaining RXC, both D-haplotype fish, clearly exhibited white on the pelvic and/or anal fins. Lack of a RXC haplotype in these two fish emphasizes that mtDNA only expresses maternal inheritance. Sequencing of the mtDNA ND2 gene essentially functioned as a fine-filter screening of all 324 samples from throughout the study landscape. This suggests that hybridization is largely limited to those locales previously suspected of harboring RBT or RXC. Recommendations based on these results include: 1) Initiate a landscape level analysis using this sample set and nuclear markers (microsatellites) to understand historic geologic and hydrologic conditions that may explain the patterns of genetic variability observed in this study; 2) While not conclusive, frequency differences among drainages suggest that there should be caution in translocating cutthroat trout among drainages; and 3) Initiate mainstem Snake River investigations to better determine the presence, location, and extent of hybridization in the river between Palisades Reservoir and Jackson Lake. xi INTRODUCTION The cutthroat trout Oncorhynchus clarki is the only trout native to Wyoming (Baxter and Stone 1995). Three recognized sub-species of cutthroat trout have been described within the state and inhabit tributaries of the Bear River (Bonneville cutthroat, O. c. utah), Colorado/Green River (Colorado cutthroat, O.c. pleuriticus), and the Yellowstone River and Snake River (Yellowstone cutthroat, O. c. bouvieri, YSC). While the differentiation among sub-species has been well documented, a morphometrically distinct fish has been identified within the Snake River system that may also be unique. A finespotted morphotype (finespotted Snake River, SRC) has been identified in the Snake River headwaters of northwest Wyoming that is a visually distinct, yet taxonomically puzzling native fish (Behnke 1992) that has been proposed for subspecies status (Baxter and Simon 1970; Behnke 1992). However, there have been no attempts to determine whether YSC and SRC in the Snake River headwaters differ with respect to ecology, morphology, and/or genetic characteristics. Though Yellowstone cutthroat are one of the more well-studied subspecies of interior cutthroat trout (see Behnke 1992; Gresswell 1988; and Gresswell 1995), the majority of work to date has been conducted in segments of large rivers or lakes of Idaho, Montana, and Wyoming outside of the Snake River basin. The majority of the research within the Snake River has described the biology and ecology of the SRC fishery in the mainstem Snake River in Jackson Hole (Hayden 1967; Wiley 1969; Hagenbuck 1970; Kiefling 1972, 1978; Harper and Farag 2002; Harper and Farag 2004). Previous genetic investigations of fish from both the mainstem and headwater tributaries were used to describe the phylogeography of the interior cutthroat trout (Murphy 1974), examine genetic differences between YSC and SRC (Loudenslager 1978), and used to compile a biological classification of native trout of western North America (Behnke 1992). Little research has occurred in the remaining tributaries (Greys River, Hoback River, Gros Ventre River, Buffalo Fork) flowing into the Snake River between Palisades Reservoir and Jackson Lake dam. The identification of the current distribution and status of populations of YSC has become a management priority for state and federal agencies due to their continued decline in distribution and abundance throughout most of the historical range. Causes for these declines include the loss of habitat due to poor land use practices, overfishing, and the introduction of non-native species that have successfully invaded and occupied YSC habitat (Behnke 1992). Concern over the status of these fish prompted a status review of YSC following a 1998 petition to list them as a threatened species under the Endangered Species Act (WGFD 1999). The YSC was eventually found to be “not warranted” for listing, but the US Fish and Wildlife Service is under court order to re-examine the data used for the initial finding and proceed with a 1-year status review. The Snake River above Palisades dam has been identified as a large, relatively intact basin that represents one of the last strongholds of YSC and the potentially unique SRC morphotype. However, the status of cutthroat trout populations and the threats to these populations have never been formally examined. This work examines three major questions that need to be answered in order to determine the status of 1 cutthroat trout in the Snake River above Palisades dam. First, are there morphometric and genetic differences between YSC and SRC that would indicate that these are unique subspecies and that these fish should be managed separately? Second, if there are no apparent subspecies differences, are there differences in the genetic structure among geographic units that would indicate separate management units within the basin? Third, given the stocking history of rainbow trout and other non-native cutthroat trout, where have populations within the basin been compromised by hybridization and where are the potential threats? We used these questions to develop the following study objectives: 1) Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions. 2) Determine morphometric and genetic differentiation between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape. 3) Describe patterns of genetic variation in cutthroat trout within and among major drainages in the study landscape. 4) Assess introgression with rainbow trout using both morphologic and genetic tools. Yellowstone Cutthroat Trout Phylogeography and Systematics Yellowstone cutthroat trout are native to the Yellowstone River and Snake River headwaters in Idaho, Montana, and Wyoming (Figure 1). Their trans-continental divide range in Montana and Wyoming likely resulted from headwater connection 20,000 to 50,000 years ago, similar to the present connectivity of Atlantic Creek and Pacific Creek at Two Ocean Pass (Behnke 1992). This connection between the Columbia and Missouri River basins remains, and fish are not restricted from inter-basin movement, even today. Yellowstone cutthroat trout became isolated in the headwaters of the Snake River following creation of Shoshone Falls (between 30,000 and 60,000 years ago). The finespotted morphotype is hypothesized to have originated from the YSC in present-day Jackson Hole during the Pinedale glacial period 15,000 to 25,000 years ago while isolated by glacially dammed lakes (Loudenslager and Kitchin 1979, Love et al. 2003). Behnke (1992) postulates that, “after several thousand years of isolation, the ancestral Yellowstone cutthroat trout and the new form, both slightly differentiated after isolation, came together again, but instead of freely hybridizing, they partitioned the Snake River headwaters environment and maintained their distinctions through reproductive isolation. Once in contact again, evolutionary mechanisms governed by natural selection probably resulted in their spotting differences.” Alternatively, continued partitioning of the riverscape after breakdown of the hypothesized barrier may depend more upon low average dispersal distance of individuals from populations (Irwin 2002). The finespotted morphotype was considered a taxonomically unidentified native fish (Behnke 1992) with a historical range limited to the Snake River headwaters of northwestern Wyoming between Palisades Reservoir 2 Approximate historical range of Snake River cutthroat trout Continental Divide Figure 1 Historical transcontinental range of Yellowstone cutthroat trout, with finespotted Snake River cutthroat trout historical range indicated. 3 and Jackson Lake, and possibly eastern Idaho. Montgomery (1995) proposed to name it a subspecies; Oncorhynchus clarki behnkei. However, the name was invalidated due to omission of a type specimen (D. Shiozawa, personal communication). The actual recognition of the SRC as a subspecies distinct from the YSC remains unresolved (Behnke 2002). The SRC and YSC morphotypes are closely related (Loudenslager and Kitchin 1979; Loudenslager and Gall 1980), and F1 progeny exhibiting intermediate spotting patterns have been observed when hatchery stocks were combined with wild populations (Behnke 1992). Behnke (1992) suggests that reproductive isolation is not complete between SRC and YSC when sympatric. His analyses have shown variation and overlap in meristic counts, and observations of intermediate spotting suggest continued gene flow. Behnke (1992) also acknowledged that the difference in spotting pattern, and observed intermediate spotting, may result from simultaneous expression of two co-dominant alleles at one locus, as shown by Skaala and Jorstad (1988) in brown trout Salmo trutta. Genetic comparisons of YSC and SRC (Leary et al. 1987, Allendorf and Leary 1988) with allozyme electrophoresis did not discern diagnostic markers at the many loci analyzed. More recent analyses by Kruse et al. (1996) of YSC in the Greybull River drainage (Missouri River drainage) of northwest Wyoming showed no consistent difference in counts of seven meristic features of fish sampled from 18 streams. Seven of the 18 sample locations were selected due to close proximity to known locations of past SRC introductions by state fishery personnel. They also compared fish among streams thought to contain “pure” YSC based on the absence of an allele (AK-1*333; common among SRC in the Snake River drainage; Wild Trout and Salmon Genetics Lab, University of Montana, Missoula), and streams where protein electrophoresis confirmed presence of the allele; its presence was assumed to be indicative of integration with stocked SRC. Relatively little recent work has occurred on the phylogenetic classification of these fishes (Shiozawa and Williams 1992), and differences in spot size and numbers remain the only means to distinguish between YSC and SRC. Yellowstone cutthroat trout have medium to large sized spots (3-5 mm diameter) that are concentrated toward the caudal peduncle (Figure 2). Snake River cutthroat trout have a profusion of smaller spots (1-2 mm diameter) that are well distributed across the side of the fish (Figure 3). Variations in these spotting patterns (i.e., fewer medium size spots more evenly distributed) are common, and suggest mixing, or possibly environmental influences, such that distinguishing between these fishes is not possible in all cases (Figure 4). Spotting patterns have been shown to correctly classify YSC and SRC (>95%) in blind tests where there was no hybridization with rainbow trout, but were not useful in identifying fish with intermediate spotting patterns (Kruse 1998). Application of recent advances in molecular genetic techniques have identified mtDNA haplotypes that distinguish populations of YSC isolated by distance (Campbell et al. 2002), and nDNA markers distinguishing YSC from the other inland cutthroat trout subspecies (Spruell et al. 2001) and rainbow trout (RBT), though the techniques have not been applied to the closely related YSC and SRC morphotypes. 4 Figure 2 Yellowstone cutthroat trout Oncorhynchus clarki bouvieri (YSC). This fish exhibits the YSC spotting pattern, with larger spots that are concentrated towards the caudal peduncle. Figure 3 The finespotted Snake River cutthroat trout Oncorhynchus clarki subspecies (SRC) remains taxonomically undescribed. This fish shows the classic SRC pattern with small well distributed spots. Figure 4 A cutthroat trout exhibiting a common intermediate spot pattern, with small to medium spots that are concentrated toward the caudal peduncle. 5 Cutthroat Trout Distribution in the Snake River Headwaters No definitive description of the historical range of cutthroat trout in the Snake River headwaters exists. Behnke (1992) hypothesizes that only YSC were historically present in tributaries of the Snake River upstream of the Gros Ventre River confluence and in headwater streams of the Gros Ventre River drainage. He speculates that the finespotted morphotype historically occupied the Snake River downstream of Jackson Lake and all tributaries downstream of the Gros Ventre River. However, our early distribution surveys suggest a pattern of headwater occupancy by YSC persists in each of the river drainages, as well as in many of the smaller tributaries to the Snake River. A 1996 habitat conservation assessment by May (1996) suggested YSC and the finespotted morphotype may be present in 100% of their historically occupied streams and lakes in the Snake River headwaters of Wyoming, and occupy additional lakes as a result of past and current stocking practices. Due to the coarse scale of analysis and use of “best available information”, caution must be exercised in interpreting May’s findings. Allendorf and Leary (1988), and Varley and Gresswell (1988), and others (Young 1995) have identified the introduction of non-native fishes as posing the greatest danger to native cutthroat trout conservation, due mainly to interbreeding, and the primary cause for decline of YSC in other portions of their range. Prior to our work, no information on genetic status of these fishes (e.g., introgression by rainbow trout) was available for our study streams. While non-native trout introductions have been widespread throughout the basin, displacement of cutthroat trout (e.g., by brook trout), and presumably introgression (e.g., with rainbow trout) are assumed to have occurred on a limited basis. Study Area Description At the broad scale this work assessed the landscape distribution and organization of cutthroat trout populations between Palisades Reservoir and Jackson Lake, Wyoming (Figure 5). The distribution surveys were conducted in all named streams, including the Greys River, Hoback River, and Gros Ventre River drainages. All named tributaries to the Snake River were surveyed upstream to Jackson Lake Dam. Mapping of mtDNA haplotypes by geographic areas or major river drainages was completed after initial analysis of samples from across the basin. Five geographic areas were identified a priori for mapping and analyses. These areas, as they generally occur from north to south, include Jackson Hole, Gros Ventre, Hoback, Snake River Canyon, and Greys. The Snake River and its tributaries were split due to the stark geomorphological break between the broad mountain valley of Jackson Hole, and the Snake River Canyon. The remaining three areas are comprised of the 6 Jack son Lake Jackson Hole Gro s Ve ntre Snake River Canyon es ad lis Pa Ho b ac k oir rv se Re Wyoming Idaho ys Gre Figure 5 Snake River headwaters study area, in northwest Wyoming (approximately 9,440 km2). The five geographic areas between Palisades Reservoir and Jackson Lake are: Jackson Hole, Gros Ventre, Hoback, Snake River Canyon, and Greys. 7 major tributary river drainages; the Salt River drainage was excluded due to being highly fragmented by water developments and not meeting the stream selection criteria (see following section). Surveys were conducted throughout the length of streams occupied by fish, including above and below natural barriers. Analyses were largely constrained to connected stream networks, except for several isolated stream segments above natural barriers in the Teton Mountains. Scale of Analysis and Geographic Sub-sampling The landscape scale approach facilitated the synthesis of geomorphic, ecological, and genetic information regarding the distribution and organization of cutthroat trout within the river drainage, watershed, stream, or stream reach. Samples were selected for inclusion in this analysis based on trout external morphology (spot patterns). Streams were selected to be representative of the five geographic areas comprised of four river drainages in the riverscape. Selection criteria allowed us to hierarchically analyze for morphological or geographic structuring from the basin scale, to the stream reach scale. Specifically, samples were selected by stream based on the following criteria: 1) Ensure variation in spotting patterns within each stream was documented by surveys throughout the occupied length of a stream; 2) There was no history of stocking, or at least recent stocking, in each stream to the extent possible; 3) Connectivity existed among all streams selected, both within and between the geographic areas; 4) Minimize spatial clustering of samples within a stream, to the extent possible, by selecting samples from throughout the occupied length of each stream; 5) Minimize spatial clustering of streams, to extent possible, by selecting streams from throughout each geographic area; 6) Ensure that streams were stratified across the five geographic areas; 7) Ensure that streams were stratified within each of the five geographic areas; 8) Include samples from each of the available age classes or size groups within each stream; 9) Include a minimum of n=30 fish from each geographic area or river drainage, where possible, that exhibit the large-sparse spotting pattern (i.e., YSC); 8 10) Segregate fish into three distinct spot pattern morphotypes: Type 1 – large-sparse (L), Type 2 – fine-dense (F), and Type 3 – intermediate (I) to 1 and 2; 11) Morphotypes present must be confirmed based on photographic records from stream surveys; and 12) Samples to be included in the analysis should be only from those streams where the large-sparse morphotype was observed. Exceptions to these criteria were allowed only in the case where number of streams with the large-sparse spotting pattern were limited, necessitating the inclusion of streams or samples within streams that were above man-made barriers to upstream movement or stocking was documented in the last 50 years. Furthermore, availability of tissue samples from cutthroat trout with photographic documentation in large rivers was inconsistent, specifically in the case of the Snake River. Samples were clustered in two areas between the Gros Ventre River confluence and Jackson Lake, and no samples were available in the southern portion of Jackson Hole or the Snake River Canyon (Figure 5). METHODS Sample Collection Systematic electrofishing surveys were completed to verify fish presence and distribution in all named streams on National Forest, National Park, and National Refuge system lands between Palisades Reservoir and Jackson Lake dam (Figure 5). Sampling was conducted with a model 12B Smith-Root battery powered backpack electrofisher. Crews consisted of one person operating the electrofisher and two netters. A single netter was employed only where the wetted width of the stream was <1.0 m. Angling or boat electrofishing surveys were conducted with a single fisher or netter on larger streams where backpack electrofishers were ineffective. Our sampling goal was to provide a reasonable probability of detecting YSC versus SRC within any one stream occupied by cutthroat trout. When sampling across an environmental gradient (e.g., a stream flowing down an elevation gradient), or the logistical demands or cost of systematic sampling approaches that of random sampling, systematic sampling may be pursued (Krebs 1999). Assuming a sampling efficiency of 0.50 for each site, a Poisson sampling distribution, and a minimum of 150 YSC in 10,000 m of stream, the probability of detection should exceed 0.80. Assuming 50 m of stream sampled at 1,000 m intervals, the expected minimum number of fish detected is: = (150/10,000 m) x 500 m sampled x 0.50, and the probability of detection no fish is given by Equation 1 as; Equation 1 P0 e = 0.02 (Zar 1999) then P(1 or more) = 0.98 (after Rieman and McIntyre 1995). 9 Stream Sample Intervals Systematic sample intervals were based on total perennial stream length on 1:24,000 topographic maps. A minimum of ten reach intervals were delineated (e.g., 5.0 km map length divided by 10 = minimum 10 sample reaches at 500-m intervals) by rounding to the nearest 50 or 100 m. Maximum interval distance regardless of total stream length is 2000 m. For streams with intervals <1,000 m, all reaches are measured using a drag tape while walking the stream bank or paced. Streams with sample intervals >1,000 m were accessed directly by road or trail without actual measuring or pacing of the stream length. One crew member generally walked the stream bank to confirm mapped sample reach breaks, while the remaining crew members drove or hiked to the next reach. Stream Sampling Protocols Single-pass electrofishing was conducted by subsampling 50-m to 100-m within each reach. Subsample length was determined by capture of a minimum of three fish >150 mm (for subspecies identification, minimum total length for YSC and SRC to develop their spotting pattern, and loss of parr marks). Sampling ceased after no fish were captured in three consecutive reaches, a barrier to upstream occupancy was encountered, headwaters were reached, or when water flow appeared insufficient to support resident fish. In each case the reason for terminating the survey was documented. Stream distance was recorded for notable physical features encountered (i.e., Forest System road or trail crossings, named and unnamed tributary confluences, water falls and high gradient riffles or cascades). Sampling began at the reach interval break, except where electrofishing conditions dictated beginning elsewhere within the reach. For example, when a reach break fell within a large beaver pond or dam complex that cannot be sampled effectively with a backpack electrofisher due to water volume, depth, or difficult access (i.e., silt bottom too difficult to safely wade), sampling began at the next accessible point upstream. A minimum 50-m sample was measured. It was often necessary to sample 75 m or 100 m in an attempt to capture a minimum of 3 cutthroat trout >150 mm. When variations in spotting pattern were observed (i.e., both YSC and SRC are identified), sampling continued up to 100 m in an attempt to capture >3 each of YSC and SRC to measure, photograph, and collect fin clips. Sampling ceased when 10 cutthroat trout >150 mm were captured. Fish Species Identification Prior to sampling a stream, the Wyoming Game and Fish Department (WGFD) stream and lake catalogue were reviewed and all previously documented fish species noted. Also, the WGFD stocking history (WGFD 1997) for the stream was reviewed, and introduced fish species noted regardless of the time since stocking. These notes provided a list of non-game species to anticipate and identify (e.g., speckled dace vs. long-nose dace vs. mountain sucker), as well as an alert to the possibility of encountering brook trout Salvelinus fontinalis (BKT), rainbow trout Oncorhynchus mykiss (RBT), rainbow trout-cutthroat trout hybrids (RXC), or cutthroat trout with confusing spot patterns (e.g., Bonneville cutthroat trout). Species were recorded in the field, as well as database entries, using the 3-letter abbreviations in Table 1. 10 Cutthroat Trout >150 mm – The cutthroat trout name arises from the red or orange slash present under each side of the lower jaw. Differences in spot size and distribution are the only recognized means to distinguish between YSC and SRC. Yellowstone cutthroat trout have medium to large sized spots (3-5 mm) that are conspicuous and rounded, and concentrated toward the caudal peduncle; color is typically yellowish brown, silvery or brassy (Figure 2). Bright golden-yellow, orange or red colors are absent (Behnke 1992; Baxter and Stone 1995). Snake River cutthroat trout have a profusion of small (1-2 mm) irregular spots that are well distributed across the side of the fish and somewhat concentrated in the caudal peduncle (Figure 3). Color is typically more yellowish brown, with orange or red on the lower fins (Behnke 1992; Baxter and Stone 1995). Cutthroat trout were identified as YSC, SRC, or CUT (delineates undetermined cutthroat trout), and comments recorded on unusual characteristics or decision criteria. Cutthroat Trout <150 mm – Young-of-the-year (age 0; 25-65 mm) and age I+ fish (approximately 80-110 mm) were often encountered and cannot be positively identified as YSC or SRC. Once confirmed as cutthroat trout (e.g., streams where juvenile or adult BKT or RBT have been captured), YOY or I+ were noted in comments and species recorded as CUT. In addition, cutthroat trout 100-150 mm were common and could not be confirmed as either YSC or SRC due to lack of a distinct spot pattern and retention of parr marks (a distinct series of dark vertical bars along each side of the fish, elongate-oval in shape, that appear as a shadow and may exhibit some reddish or brown coloration). These fish were recorded as CUT with comments on the characters resulting in no subspecies determination. Rainbow Trout – Rainbow trout typically are dark green to blue-green dorsally, with silvery sides, and a distinct bright red or pink lateral stripe. Small irregular black spots are dense on the head, body and fins. The paired fins have white margins. The cutthroat “slash” may be present, though typically less conspicuous in rainbow trout or RXC hybrids. Introgression with rainbow trout, though rare in the Snake River headwaters, is possible. Rainbow trout are known to be present in the Gros Ventre River below Lower Slide Lake, Stump Lake and Fawn Creek in the Greys River drainage, and in Rainbow Lake and the headwaters of the South Fork of the Buffalo Fork River where they have been stocked in the past. A good field identification feature of hybridization with cutthroat trout is white margins along the leading edge of the pectoral, pelvic or anal fins (Baxter and Stone 1995); white fin margins may be accompanied by profuse spotting on the head. More difficult to assess in the field are the presence of basibranchial teeth on the vomer (a characteristic of cutthroat trout). Such hybrids were recorded as RXC and the characters suggesting hybridization noted. 11 Table 1 Common and scientific names1 of fishes and amphibians in the Snake Headwaters basin of Wyoming, and species abbreviations as identified by the Wyoming Game and Fish Department. Species ID BHS BKT BNT Native Game Common Name Genus Species Subspecies Drainage2 Fish Catostomus discobolus bluehead sucker N 1,4,7 Salvelinus fontinalis brook trout Y Salmo trutta brown trout Y Bonneville (Bear River) Oncorhynchus clarkii utah BRC cutthroat trout Y 9 Colorado River Oncorhynchus clarkii pleuriticus CRC cutthroat trout Y 4,7 Oncorhynchus clarkii CUT cutthroat trout Y Pimephales promelas FHM fathead minnow N 2,3,5,6 Oncorhynchus mykiss aguabonita GDT golden trout Y Thymallus arcticus GRL grayling Y 2 Poecilia reticulata GUP guppy N Oncorhynchus nerka KOE kokanee Y Salvelinus namaycush LAT lake trout Y Rhinichthys cataractae LND longnose dace N 1,2,3,5,6,8 Snyderichthys copei LSC leatherside chub N 1,9 Cottus bairdii MSC mottled sculpin N 1,4,7,9 Catostomus platyrhynchus MTS mountain sucker N 1,2,4,7,8,9 williamsoni MWF mountain whitefish Prosopium Y 1,2,3,4,7,8,9 NOD No Data/Unknown N OOO No fish Present N Cottus beldingii PSC Paiute sculpin N 1,9 Oncorhynchus mykiss RBT rainbow trout Y cutbow RXC (RBT x CUT) Y Richardsonius balteatus RSS redside shiner N 1,9 Rhinichthys osculus SPD speckled dace N 1,4,7,9 splake SPK (BKT x LKT) Y finespotted Snake River Oncorhynchus clarkii subspecies SRC cutthroat trout Y 1 tiger trout TGT (BKT x BNT) Y TRT any trout Y Gila atraria UTC Utah chub N 1,9 Catostomus ardens UTS Utah sucker N 1,9 Yellowstone Oncorhynchus clarkii bouveri YSC cutthroat trout Y 1,2,3,8 1 Sources included Nelson et. al. 2004. Baxter and Stone 1995, and Behnke 1992. 2 Drainage Code: 1 - Snake River; 2 - Big Horn River, Shoshone River, Wind River; 3 - Powder River; 4 Green River; 5 - North Platte River; 6 - Little Missouri River, Cheyenne River, Niobrara River, Belle Fouche River; 7 - Little Snake River; 8 - Yellowstone River; 9 - Bear River. 12 Fish Metrics, Photographs, and Tissue Samples Up to 10 cutthroat trout >150 mm were measured and photographed in each sample reach; a caudal fin clip is collected from all photographed cutthroat trout and RBT (and all RBT or CUT exhibiting characters of hybridization). Fish were measured and photographed lying on their right side, dorsal fin up, and facing to the left, using a standardized measuring board. Total length was recorded to the nearest millimeter, and weight to the nearest 5 gm. After photographing the fish, a caudal clip was collected. Fin clips were >5 mm in diameter, although this proved difficult for fish <175 mm. In those cases, not more than one-quarter of the caudal fin was removed. Photographs and fin clips were identified by a common alpha-numeric code as follows: SA = Salt subbasin; GH = Greys-Hoback subbasin; GV = Gros Ventre subbasin; SH = Snake headwaters subbasin; Year = 98, 99, 00, 01, 02, or 03; Camera = 01, 02, 03; 1, 2, 3….. k = sequential number of photograph during a sample year with a given camera. For example, the 25th fish collected in the Greys-Hoback subbasin using camera #3 during 2002 was identified as ‘GH020325’. The alpha-numeric was recorded on the data sheet for the respective fish, and on the fin clip bottle. Each bottle was labeled with the date, stream name, sample reach distance, and field identification as SRC, YSC, or CUT. Genetic Analysis Tissue samples were collected from throughout the study area as described above between 1999 and 2003, and selected for inclusion based on the criteria as stated previously. Additional samples from the Snake River collected in 2004 by WGFD personnel were included upon request; those samples were screened specifically for rainbow-cutthroat trout hybrids. The approximate number of samples available for this and subsequent analyses are presented in Appendix A, Table 18. Extraction of DNA Total genomic DNA was extracted from approximately 1,280 samples (Appendix A Table 19) using a salting out technique adapted from Sunnucks and Hales (1996), and DNA quality and quantity were assessed using 0.7% agarose gels with appropriate size (100-base-pair [bp] ladder) and concentration ( Hind III digest) standards. Samples were diluted to approximately 10 ng/ul in 1X Te buffer (10 mM Tris, 0.1 mM EDTA; pH 8.0). The DNA extractions are located at Utah State University, archived in a -80°C freezer, and available for future research. 13 Methods by Objective Objective 1 – Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions Primer design and optimization for mitochondrial sequence data - An existing set of polymerase chain reaction (PCR) primers (ND12L and ND12H; Table 2; Toline et al. 1999) was available for amplification of an ~3,500 bp region of the mitochondrial NADH gene (including portions of subunits 1 and 2; ND1-2) in trout. Amplicons produced using these primers have previously been used in restriction fragment length polymorphism (RFLP) analysis in cutthroat and rainbow trout (Campbell et al. 2002; Toline et al.1999). We sought to sequence this entire region in a subset of fish from our study landscape, identify the region containing the most variation, and design new primers to amplify this (these) smaller region(s). We chose this approach because sequence data can be more informative than RFLP data in assessing phylogeographic relationships among populations. Our sample subset included sixteen samples representing the four major river drainages in the study area (Greys R, Gros Ventre R, Hoback R, and Snake R) (Table 3). Streams within drainages, and individuals within streams, were selected based on the presence of L, F, and I morphotypes. A series of internal primers developed by the Idaho Fish & Game (IDFG) Eagle Genetics Laboratory (Table 2) and our laboratory was used to obtain sequences from the ND1-2 region. Contiguous sequences for these 16 individuals were constructed and aligned using DNAStar software (Lasargene, Inc.). In this subset of fish, 15 polymorphic sites were identified in the ~3,500 bp ND1-2 region. Ten of these sites were concentrated in a 670-bp region of the ND2 gene. We designed primers and optimized conditions for the amplification of this region using only two PCR primers (Table 2). Amplification of the mitochondrial DNA ND2 gene region – An approximately 1,100 base-pair (bp) amplicon containing the NADH dehydrogenase 2 (ND2) gene region of the mitochondrial genome was amplified using polymerase chain reaction (PCR). Primers specific for the ND2 gene region – (NDintF4) TAA GCT TTC GGG CCC ATA CC and (NDvarR) GCT TTG AAG GCT CTT GGT CT – were purchased from Integrated DNA Technologies, Inc (Coralville, IA). Each 25-L PCR reaction contained 20-50 ng of extracted DNA template, 1 X PCR buffer, 0.2 mM deoxynucleotide triphosphates (dNTPs), 2.5 mM MgCl2, 5.0 M of each primer, and 1.25 units (U) of Taq polymerase. The reaction was denatured at 95o C for 2 min, followed by 30 cycles of 94o C for 1 min, 58o C for 1 min, and 72o C for 1 min 20 sec, with a final 10-min extension at 72o C. The entire amplified product was sent to the Nevada Genomic Center (NGC) at the University of Nevada – Reno to be purified, quantitated, and sequenced. Amplicons were purified using a Qiagen MinElute filter plate on the Qiagen BioRobot 3000, and quantified with a fluorescent nucleic acid stain (PicoGreen®) and read on a Labsystems Fluoroskan Ascent fluorescence plate reader. Using the primers described above, sequencing reactions were performed from both ends of the 14 Table 2 Polymerase chain reaction (PCR) primers used to amplify and sequence the ND1-2 region in cutthroat trout. Unpublished primer sources are noted: IDFG = Idaho Fish & Game Eagle Fish Health Lab; USU = Utah State University. Primer Name Sequence Source ND12L 5’ GCCTCGCCTGTTTACCAAAAACAT Toline et al. 1999 ND12H 5’ CCGGCTCAGGCACCAAATAC Toline et al. 1999 NDintR1 5’ CCTGATCCAACATCGAGGT IDFG NDIntF2 5’ ACCTCGATGTTGGATCAGG IDFG NDIntR3 5’ GCGTACTCGGCTAGGAAAAA IDFG NDIntF4 5’ GGGCAGTGGCACAAACTATT IDFG NDIntR5 5’ GGTATGGGCCCGAAAGCTTA IDFG NDIntF6 5’ TAAGCTTTCGGGCCCATACC IDFG NDIntR7 5’ GGGTCGGGGATTTAGTTCAT IDFG NDIntF8 5’ ATGAACTAAATCCCCGACCC IDFG Jess16sF 5’ ACCAAAAACATCGCCTCTTG USU JesstRNAR 5’ GGGGGAAAGTAGATGGATGC USU NDvarF 5’ GAC AAA AAC TCG CAC CCT TC USU NDvarR 5’ GCT TTG AAG GCT CTT GGT CT USU amplicons with an ABI BigDye Terminator Cycle Sequencing Ready Reaction Kit v3.1, and the reactions are then run on an ABI3730 DNA Analyzer. Two sequencing reactions, one each forward and reverse, were sufficient to provide complete coverage of the ND2 gene. For each individual, these two sequences were used to assemble a contiguous sequence with DNASTAR SeqMan software (Lasergene). These contiguous sequences were aligned with DNASTAR MegAlign software (Lasergene), and the aligned sequences trimmed to a total length of 945 bp. Primer selection and optimization for nuclear microsatellite data – Microsatellite loci from three different sources were screened for utility in cutthroat trout from the study landscape (Table 4): 1) LHC loci (10 loci developed for use in Lahontan cutthroat trout [Peacock et al. 2004]); 2) IDFG loci (6 loci developed for a mix of species and used by the Idaho Fish and Game Department, Eagle Genetics Laboratory, to assess Yellowstone cutthroat trout population genetic structure in Idaho; Rexroad et al. 2002; Wenberg and Bentzen 2001; Nelson and Beacham 1999; Olsen et al. 1998; Sakamoto et al. 1994; Condrey and Bentzen 1988); 3) RGC loci (14 unpublished loci developed for use in Rio Grande cutthroat trout for the New Mexico Department of Game and Fish [K.Jones, Genetic Identification Services]). These loci were initially assessed for their ability to produce an amplicon of the 15 Table 3 Sample subset used to assess landscape-scale sequence variation in the mitochondrial ND1-2 region and to design internal primers to capture this variation. Drainage/Stream Location (m) Species Length (mm) Weight (gm) Sample ID Cabin Creek 6,000 SRC 153 44.0 GH-01-02-06 Cabin Creek 6,000 CUT 161 40.0 GH-01-02-08 Flat Creek 48,000 CUT 192 64.0 GH-02-01-60 Flat Creek 48,000 SRC 168 48.0 GH-02-01-61 Pacific Creek 42,000 CUT 230 112.0 SH-03-02-467 Pacific Creek 32,000 SRC 238 110.0 SH-03-02-494 North Fork Fish Creek 8,000 SRC 360 410.0 GV-99-35-18 North Fork Fish Creek 8,000 CUT 172 55.0 GV-99-35-19 Bondurant Creek 2,000 SRC 206 86.0 GH-01-05-29 Bondurant Creek 2,000 YSC 194 71.0 GH-01-05-30 Bull Creek 3,000 YSC 199 105.0 GH-01-15-12 Bull Creek 36,00 SRC 190 90.0 GH-01-15-14 Blind Trail Creek 7,000 SRC 219 108.0 GH-00-24-08 Blind Trail Creek 7,000 YSC 225 104.0 GH-00-24-10 Flat Creek 3,000 SRC 184 73.0 GH-00-17-08 Flat Creek 3,500 YSC 218 81.0 GH-00-14-17 SNAKE RIVER GROS VENTRE RIVER HOBACK RIVER GREYS RIVER 16 Table 4 Polymerase chain reaction (PCR) primers used to amplify and assess polymorphism at nDNA microsatellite loci in cutthroat trout. Unpublished primer sources are noted by place of origin: GIS = Genetic Identification Services. Microsatellite Group/Locus Source PCR Successfully Optimized Polymorphic in Study Samples Protocols Developed Peacock et al. 2004 Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. YES NO NO YES NO YES YES NO NO NO NO NO NO NO NO YES YES NO NO NO NO NO NO YES NO YES YES NO NO NO Sakamoto et al. 1994 Condrey and Bentzen 1988 Olsen et al. 1998 Rexroad et al. 2002 Nelson and Beacham 1999 Wenberg and Bentzen 2001 NO YES YES YES YES YES NO YES YES YES YES NO NO YES YES YES YES YES GIS Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. Ibid. YES YES YES YES YES YES YES YES YES YES YES YES YES NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA LHC GROUP OCH5 OCH6 OCH9 OCH10 OCH11 OCH13 OCH14 OCH15 OCH16 OCH17 IDFG GROUP Fgt3 Ocl1 Ogo4 Omm1036 Ots107 Ssa85 RGC GROUP G20 H12 H18 H114 H118 H126 H204 H220 J3 J14 J103 J132 K216 17 expected size under standard PCR conditions. Limited protocol optimization was attempted for loci producing visible amplicons of the appropriate size (based on 1% agarose gel electrophoresis, with ethidium bromide staining and visualization under UV light). Fluorescent dye-labeled primers were ordered for LHC and IDFG primers yielding reliable amplification, and amplicons were assessed for polymorphism in the same subset of cutthroat trout samples as described above (Table 3). The trial subset varied from locus to locus. Optimization of RGC loci was suspended after confirmation of amplicons, and therefore, no polymorphism or protocol information is presented. Objective 2a – Determine morphological differences between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape We used the only recognized morphological pattern that identifies SRC from YSC, spot size and distribution, as an initial filter for field determinations during sample collection. A second level filter was applied that we based on further spot pattern analysis of our photographic records (scanned 35 mm slide or digital). This classification of cutthroat trout resulted in fish being binned as one of three distinct spot pattern morphotypes: Type 1 – large-sparse (L), Type 2 – fine-dense (F), and Type 3 – intermediate (I) to 1 and 2. Intermediate fish could not be clearly binned as 1 or 2 due to exhibiting shared characteristics of YSC and SRC as described in the Fish Species Identification section. The photograph of each specimen was reviewed and assigned a morphotype, prior to inclusion of its tissue sample for DNA extraction. A 6-cell grid was visualized on the fish as follows (after Quadri 1959 as adapted by Kruse 1998): 1) Anterior insertion of adipose fin below the lateral line; 2) Same as area 1 except above the lateral line; 3) Behind anterior insertion of the dorsal fin below the lateral line back to boundary of area 1; 4) Same as three except above the lateral line back to boundary of area 2; 5) Below lateral line forward from boundary of area 3 to opercle; and 6) Same as area 5 except above the lateral line forward from boundary of area 4. Fish assigned to morphotype 1 had spots predominantly 3-5 mm in diameter, and concentrated in cells 1-2 and 4. Those assigned to morphotype 2 had spots predominantly 1-2 mm in diameter, and typically well distributed throughout at least 5 of the 6 cells; cell 5 typically had comparatively fewer spots. Fish assigned to morphotype 3 generally exhibited an intermediate spot size (2-4 mm diameter) with spots either well distributed or concentrated toward the caudal peduncle. Spotting patterns were also used by Kruse (1998) to accurately classify genetically pure YSC and SRC, but were of little utility in identifying hybrid individuals (YSCxSRC). However, Kruse found that visual field classifications based on spotting patterns, and the presence of throat slashes and white fin margins or tips performed better than discriminant models with either meristic features or spotting patterns in identifying genetically pure cutthroat trout and RBT, as well as indicating genetic introgression (i.e., rainbow-cutthroat hybrids [RXC]). 18 Objective 2b – Determine genetic differentiation between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape Individual fish were assigned a morphotypic category (fine-dense, F; intermediate, I; large-sparse, L; or anamolous) as described in Objective 2a methods above. Photographs of these fish, and their assigned categories, are provided in (Appendix B, CD - name files). Mitochondrial ND2 sequences (~1,100 bp) were obtained for 324 fish representing all major drainages and all morphotypic categories (Appendix B, CD - name files), following the protocol developed in Objective 1 (see Results section, below). These sequences were trimmed to 945 bp and aligned using DNAStar (Lasargene, Inc.) software. Individual sequences were collapsed into 13 distinct haplotypes. Genetic differentiation between morphotypic categories was assessed by comparing the distribution of haplotypes among these categories, first by pooling all samples across the entire study area and second by comparing morphotypic groups within geographic areas (Jackson Hole, Gros Ventre, Hoback, Snake River Canyon, and Greys; Figure 5). Comparisons among morphotypic groups were based on the average sequence divergence among all pairs of individuals within and between groups, expressed as the number or proportion of pairwise nucleotide differences, using MEGA v3.0 software (Kumar et al. 2004). Standard errors for these distances were estimated in MEGA using 500 bootstrap replicates. Objective 3 – Describe patterns of genetic variation in cutthroat trout within and among major drainages in the study landscape Five geographic areas were represented in our sampling scheme (Figure 5): Jackson Hole (n=139), Gros Ventre (n=62), Hoback (n=40), Snake River Canyon (n=26), and Greys (n=57). Because genetic differentiation among morphotypes was not detected (see Results), morphotypes were pooled for the purpose of assessing differentiation among drainages. Genetic differentiation among drainages was assessed using three methods: a) average pairwise nucleotide differences within and between drainages was characterized as described above for morphotypic groupings, using MEGA software (Kumar et al. 2004); b) A Chi-square test of the distribution of haplotypes (without regard to haplotype sequence divergence) among drainages was performed using DnaSP software (Rozas et al. 2003); and c) Genetic differentiation among pairwise drainages was characterized via the GST statistic (Nei 1987; Hudson et al. 1992) using DnaSP software (Rozas et al. 2003). Relationships among haplotypes were assessed by a) constructing a neighborjoining dendrogram based on the number of nucleotide differences with MEGA software (Kumar et al. 2004), and b) constructing a haplotype network, based on statistical parsimony, using TCS software v1.18 (Clement et al. 2000). 19 Genetic diversity within drainages was assessed by estimating nucleotide diversity (π), haplotype diversity (Hd), and enumerating haplotypes, using DnaSP software (Rozas et al. 2003). Objective 4 – Assess introgression with rainbow trout using both morphologic and genetic tools For field identification we used the morphological features described in the Fish Species Identification section for rainbow trout, and the following as the primary indicator of rainbow trout hybridization with cutthroat trout (Kruse 1998; Baxter and Stone 1995): white margins along the leading edge or tips of the pectoral, pelvic or anal fins. Genetic identification of rainbow trout and introgression with cutthroat in the study landscape was assessed with the mitochondrial ND2 gene region and protocol developed in Objective 1. Putative rainbow trout and rainbow-cutthroat hybrid sequences were trimmed, aligned, and included in the haplotype collapse described in Objective 2b. Relationships to the cutthroat trout haplotypes we identified were assessed by inclusion of each rainbow trout and rainbow-cutthroat haplotype in the neighbor-joining dendrogram we constructed; the rainbow trout ND2 sequence was obtained from GenBank (http://www.ncbi.nlm.nih.gov/Genbank/). RESULTS Survey Results Our distribution surveys conducted in the Snake River headwaters since 1998 indicate present occupancy of cutthroat trout in >95% of streams and >90% of stream length inhabited by trout. This includes 393 streams between Palisades Reservoir and Jackson Lake dam, with approximately 2,500 sample locations in more than 2,260 km of habitat surveyed (Table 5). Cutthroat trout were found in 294 streams in 1,483 km of habitat (Table 6). Nine rainbow-cutthroat hybrids were found in the Greys (2), Hoback (1), and Gros Ventre (6) areas during the survey. They were present in <14 km of habitat (Table 6), and do not appear to have displaced native cutthroat trout in any of the sample streams. Brook trout were found in all areas (Table 6). Brook trout may have displaced cutthroat trout from 13 of the 81 streams they occupied, totaling 17 km of habitat. Yellowstone cutthroat trout (large spotted morphotype) were found in considerably fewer streams (102 streams, 277km) and in fewer locations than Snake River cutthroat trout (fine spotted morphotype; 258 streams, 1,249 km, Table 7). 20 Table 5 Summary by river drainage for numbers of streams and stream reaches, and stream length (km) surveyed for cutthroat trout presence/absence between 1998 and 2003 in the Snake River headwaters of northwest Wyoming. River drainages are listed as they flow into the Snake River proceeding upstream from Palisades Reservoir. Streams1 Reaches Length (km) Snake River 2 125 803 790 Greys River 93 678 500 Hoback River 64 393 385 Gros Ventre River 110 611 553 Buffalo Fork River 3 1 16 32 393 2,501 2,260 Drainage Total 1 Includes main stem and tributaries unless otherwise noted. Largely tributaries; only 14.0 km surveyed on main stem Snake River between Palisades Reservoir and Jackson Lake dam. 3 Includes no tributaries. Only 32.0 km of Buffalo Fork River were surveyed between Snake River confluence and bridge on Forest Road 30050. 2 Table 6 Number of streams with cutthroat, brook, and rainbow trout present and the stream length (km) occupied, based on presence/absence surveys between 1998 and 2003 in the Snake River headwaters, Wyoming. Cutthroat Trout 1 2 Drainage Brook Trout 2 Hybrid Trout 2 All Trout Stream Length Stream Length Stream Length Stream Length Snake River 87 410 42 123 - - 96 489 Greys River 82 373 8 26 1 0.83 83 380 Hoback River 3 45 268 8 42 1 2.50 47 289 Gros Ventre River3 79 408 22 71 2 10.0 80 437 Buffalo Fork River 1 24 - - - - 1 24 294 1,483 80 261 4 13.33 307 1,619 4 Total 1 Includes YSC, SRC, and unidentified juvenile (<150 mm) or adult (>150 mm) cutthroat trout. Occupied stream length for species indicated is total whether allopatric or in sympatry with other species given. 3 Only rainbow-cutthroat trout hybrids were captured. 4 Includes no tributaries. Only 32.0 km of Buffalo Fork River were surveyed between Snake River confluence and bridge on Forest Road 30050. 2 21 Table 7 Presence of Yellowstone cutthroat trout (large spotted morphotype) and Snake River cutthroat trout (fine spotted morphotype) in streams surveyed, and stream length (km) occupied in the Snake River headwaters, Wyoming. Yellowstone cutthroat trout (Morphotype L) Drainage Snake River cutthroat trout (Morphotype F) Yellowstone and Snake River Morphotypes Streams Length Streams Length Streams Length Snake River 33 119 75 304 33 92 Greys River 17 21 77 328 16 16 Hoback River 13 28 37 230 12 24 Gros Ventre River 38 103 68 363 36 87 Buffalo Fork River 1 6 1 24 1 6 102 277 258 1,249 98 225 Total Both large and fine spotted morphotypes were co-located in 98 streams, representing 225 km. Yellowstone cutthroat trout occur almost exclusively in sympatry with Snake River cutthroat trout. Genetic Structuring Results by Study Objective Objective 1 – Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions Amplification of the mitochondrial DNA gene region – A pair of PCR primers were developed for use in upper Snake River cutthroat trout genetic analyses. These primers reliably amplify a ~1,100 bp region of the ND2 mitochondrial gene, and can be used both for amplification and sequencing: Forward Primer: NDintF4 5’ GGGCAGTGGCACAAACTATT (IDFG) Reverse Primer: NDvarR 5’ GCTTTGAAGGCTCTTGGTCT PCR Reaction (25 ul volume): 4.0 ul of 5.0 mM dNTPs (final concentration 0.2 mM for each dNTP); 2.5 ul of 10x buffer; 2.5 ul of 25.0 mM MgCl; 0.5 ul of each primer (10 uM stock); 0.25 ul of Taq polymerase (5.0 U/ul stock); 2.5 ul (20.0ng) template. 22 PCR Thermal Profile: Hold @ 95o C for 2 min; 30 cycles of – 94o C for 1 min.,58oC for 1 min., 72o C for 1 min. 20 sec.; Final extension @ 72o C for 10 min. Amplification of the nuclear DNA gene regions – Six polymorphic microsatellite loci were identified for use in Snake River headwaters cutthroat trout genetic analyses. The primer pairs reliably amplified these loci, and allele sizes ranged from approximately 100 to 400 bp. Multiplexing (simultaneous amplification) of several loci was not attempted pending determination of polymorphism for the RGC primers: Locus: OCH 13 Forward Primer: 5’ GGA GGT GAT TCT ATG GGT AAA T Reverse Primer: 5’ CAG ATG GGC ACT TAG ATT GTT Label: HEX green FILTER SET D Clone Length: 144-262 bp Locus: OCH 14 Forward Primer: 5’ CGG GCT ATA TGA AGG TGA TCC Reverse Primer: 5’ GCT ACG CAA ATG AAC AAA CCA Label: TAMN- yellow FILTER SET D Clone Length: 263-419 bp PCR Reaction OCH 13 and 14 Reagent ddH20 dNTP (ea.) PCR buffer MgCl2 primer forward primer reverse Taq template (1:10) Stock Final 1.25 mM 10X 25 mM 10 mM 10 mM 5 units/μL 20.0 ng/μL 0.20 mM 1X 1.8 mM 1 mM 1 mM 20.0ng Total Volume x1 (μL) 9.33 1.2 (0.3 ea.) 1.5 1.08 0.345 0.345 0.2 1.0 15.0 μL PCR Thermal Profile OCH 13 and 14: 30 cycles of – 95oC for 30s, 58oC for 1 min 45s; Final extension @ 72o C for 10 min. Locus: Ocl1 Forward Primer: 5’ ACT ACT AAC CAG CCC ACC ACC C Reverse Primer: 5’ AGA CAG AGA GGG AGG GAA GC Label: HEX green FILTER SET D Clone Length: 100-160 bp 23 PCR Reaction Ocl 1 Reagent ddH20 dNTP (ea.) PCR buffer MgCl2 primer forward primer reverse Taq BSA template (1:10) Stock Final 1.25 mM 10X 25 mM 10 mM 10 mM 5 units/μL 10 mM 20.0 ng/μL 0.20 mM 1X 2.5 mM 0.2 mM 0.2 mM 0.038 mM 0.8 mM 20.0ng Total Volume x1 (μL) 11.25 1.2 (0.3 ea.) 2.0 2.0 0.4 0.4 0.15 1.6 1.0 20.0 μL PCR Thermal Profile Ocl 1: 33 cycles – 95oC for 30s, 63oC for 30s, 72oC for 30s; Final extension @ 72o C for 10 min. Locus: Ogo 4 Forward Primer: 5’ GTC GTC ACT GGC ATC AGC TA Reverse Primer: 5’ GAG TGG AGA TGC AGC CAA AG Label: HEX green FILTER SET D Clone Length: 120-130 bp PCR Reaction Ogo 4 Reagent ddH20 dNTP (ea.) PCR buffer MgCl2 primer forward primer reverse Taq BSA template (1:10) Stock Final 1.25 mM 10X 25 mM 10 mM 10 mM 5 units/μL 10 mM 20.0 ng/μL 0.20 mM 1X 2.5 mM 0.5 mM 0.5 mM 0.038 mM 0.8 mM 20.0ng Total Volume x1 (μL) 10.05 1.2 (0.3 ea.) 2.0 2.0 1.0 1.0 0.15 1.6 1.0 20.0 μL PCR Thermal Profile Ogo 4: 33 cycles – 95oC for 30s, 59oC for 30s, 72oC for 30s; Final extension @ 72o C for 10 min. Locus: Omm 1036 Forward Primer: 5’ TGT AGC AGG TGA GAA TAC CCA Reverse Primer: 5’ CAC CAT CTC CAT CCT AGG C Label: HEX green FILTER SET D Clone Length: TBD 24 PCR Reaction Omm 1036 Reagent ddH20 dNTP (ea.) PCR buffer MgCl2 primer forward primer reverse Taq BSA template (1:10) Stock Final 1.25 mM 10X 25 mM 10 mM 10 mM 5 units/μL 10 mM 20.0 ng/μL 0.20 mM 1X 2.5 mM 0.5 mM 0.5 mM 0.038 mM 0.8 mM 20.0ng Total Volume x1 (μL) 10.05 1.2 (0.3 ea.) 2.0 2.0 1.0 1.0 0.15 1.6 1.0 20.0 μL PCR Thermal Profile Omm 1036: 33 cycles of – 95oC for 30s, 59oC for 30s, 72oC for 30s; Final extension @ 72o C for 10 min. Locus: Ots 107 Forward Primer: 5’ ACA GAC CAG ACC TCA ACA Reverse Primer: 5’ ATA GAG ACC TGA ATC GGT A Label: HEX green FILTER SET D Clone length: 160-225bp PCR Reaction Ots 107 Reagent ddH20 dNTP (ea.) PCR buffer MgCl2 primer forward primer reverse Taq BSA template (1:10) Stock Final 1.25 mM 10X 25 mM 10 mM 10 mM 5 units/μL 10 mM 20.0 ng/μL 0.20 mM 1X 2.5 mM 0.2 mM 0.2 mM 0.038 mM 0.8 mM 20.0ng Total Volume x1 (μL) 11.25 1.2 (0.3 ea.) 2.0 2.0 0.4 0.4 0.15 1.6 1.0 20.0 μL PCR Thermal Profile Ots 107: 33 cycles of – 95oC for 30s, 50oC for 30s, 72oC for 30s; Final extension @ 72o C for 10 min. 25 Objective 2b – Determine genetic differentiation between the two morphotypes of cutthroat trout (YSC & SRC) in the study landscape Genetic differentiation among morphotypes was not apparent, either within drainages or pooling across the entire study area (Tables 8-13). Differences in haplotypic composition among groups were likely due to sample size differences or stochastic sampling error (Figures 6 -12). There was no evidence that the morphotypic groups represent distinct lineages. Table 8 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the upper Snake River drainage, Wyoming. Samples were pooled across all drainages. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype 0.005 (0.001) 5.125 (1.362) 4.740 (1.320) 0.005 (0.001) 0.005 (0.001) 0.004 (0.001) 4.135 (1.276) 0.005 (0.001) 4.964 (1.387) 4.580 (1.265) 0.005 (0.001) 4.926 (1.276) Table 9 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Jackson Hole segment of the Snake River, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype 0.003 (0.001) 3.26 (1.034) 2.608 (0.900) 0.003 (0.001) 0.003 (0.001) 0.002 (0.001) 2.067 (0.929) 0.002 (0.001) 2.496 (0.856) 1.993 (0.881) 0.002 (0.001) 1.888 (0.87) 26 Table 10 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Gros Ventre River drainage, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype 0.003 (0.001) 2.821 (0.846) 2.865 (0.802) 0.003 (0.001) 0.004 (0.001) 0.003 (0.001) 2.955 (0.895) 0.003 (0.001) 3.429 (0.970) 3.282 (0.931) 0.004 (0.001) 3.757 (1.090) Table 11 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Hoback River, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype 0.007 (0.002) 6.533 (1.741) 5.540 (1.431) 0.006 (0.002) 0.006 (0.002) 0.006 (0.002) 5.610 (1.488) 0.006 (0.002) 5.462 (1.398) 5.685 (1.455) 0.006 (0.002) 5.846 (1.543) Table 12 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Snake River Canyon segment of the Snake River, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype Large Spotted Morphotype n/a n/a 0.001 0.002 (0.001) Fine Spotted Morphotype 1.286 (0.422) 0.003 (0.001) 2.571 (0.804) 0.003 (0.001) Intermediate Morphotype 1.857 (0.579) 2.673 (0.836) 0.003 (0.001) 3.275 (0.970) 27 Table 13 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal) and between morphotypic groups of cutthroat trout in the Greys River, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype Large Spotted Morphotype Fine Spotted Morphotype Intermediate Morphotype 0.006 (0.002) 6.095 (1.687) 5.476 (1.494) 0.006 (0.002) 0.006 (0.002) 0.005 (0.001) 4.915 (1.426) 0.005 (0.001) 5.328 (1.440) 4.600 (1.302) 0.005 (0.001) 4.559 (1.298) 28 D J YSC L K C B M H CLADE 1 ? G ? ? BRC E– ? ? ? I Large Spotted Morphotype Fine Spotted Morphotype F Intermediate Morphotype ? Anomalous Morphotype =5 =1 A CLADE 2 Figure 6 Occurrence of cutthroat trout morphotypes within mitochondrial haplotypes A-M. YSC is a single occurrence haplotype from Yellowstone Lake; BRC is Bonneville cutthroat trout. Haplotype network was produced using statistical parsimony. 29 MORPHOTYPES All Drainages Ho le Ja ck so n Ho le n = 275 Fine-Dense kso n ntre Jac Large-Sparse Gros Ve Intermediate er Riv ke yon a Sn an C Ho b ac HAPLOTYPES All Drainages k n=324 ys Gre A B C D E F G H I J K L M Figure 7 Frequencies of three morphotypes and thirteen haplotypes in the Snake River headwaters study landscape. Occurrence of morphotypes was similar throughout each of the five geographic areas, whereas four haplotypes were dominant. 30 MORPHOTYPES Jackson Hole Large-Sparse Fine-Dense n = 93 Intermediate HAPLOTYPES Large-Sparse Morphotype ific ac Cr n = 11 P Leigh Canyon Paintbrush Canyon e ak Sn R South Fork Spread Cr Cascade Cr Fine-Dense Morphotype A n = 57 B Middle Fork Ditch Cr C Death Canyon D ke R H Sna J M Flat Cr Intermediate Morphotype n = 25 Flow Figure 8 Displays locations of streams in Jackson Hole from which samples were selected. Frequencies of the three morphotypes are on the upper left. Haplotype frequencies by specific morphotype are on the right. 31 HAPLOTYPES Large-Sparse Morphotype n = 13 MORPHOTYPES Gros Ventre n = 62 Large-Sparse Fine-Dense Moccasin Cr ork Fi s R Calf Cr No rt hF e ak Sn hC r Intermediate Gros Ventre R Papoose Cr Flow Park Cr Tepee Cr Fine-Dense Morphotype n = 28 A B C D n tre Gros Ve R Raspberry Cr Strawberry Cr H K L Intermediate Morphotype n = 21 Figure 9 Displays locations of streams in the Gros Ventre River drainage from which samples were selected. Frequencies of the three morphotypes are on the upper right. Haplotype frequencies by specific morphotype are on the left. 32 HAPLOTYPES Large-Sparse Morphotype n=6 A Boulder Cr B e ak Sn D R J Bull Cr Flow Hoback R MORPHOTYPES Hoback Dell Cr Kerr Cr Fine-Dense Morphotype n = 40 n = 21 Large-Sparse Fine-Dense Intermediate r nt C ura d n Bo c ba Ho kR Intermediate Morphotype n = 13 Figure 10 Displays locations of streams in the Hoback River drainage from which samples were selected. Frequencies of the three morphotypes are on the lower left. Haplotype frequencies by specific morphotype are on the right. 33 HAPLOTYPES Large-Sparse Morphotype n=5 A D I L Sn ak eR North F o So rk Fall uth F s Hor Cr ll Fa ork r eC Cr r nC bur o C Ho ba ck Cab in R HAPLOTYPES Fine-Dense Morphotype Cr n=7 Snake Flow anyon River C MORPHOTYPES Snake River Canyon HAPLOTYPES Intermediate Morphotype n = 26 n = 14 Large-Sparse Fine-Dense Intermediate Figure 11 Displays locations of streams in the Snake River Canyon from which samples were selected. Frequencies of the three morphotypes are on the left. Haplotype frequencies by specific morphotype are on the right. 34 eR Snak Little Greys R Flow HAPLOTYPES Large-Sparse Morphotype Greys R Ste er C r n=7 Blind l Cr Trai Fine-Dense Morphotype n = 30 Unnamed Tributary A B D F Upper Cabin Cr H North Three Forks Cr MORPHOTYPES Greys Intermediate Morphotype ys Gre n = 54 R Large-Sparse n = 17 Fine-Dense Intermediate Flat Cr Figure 12 Displays locations of streams in the Greys River drainage from which samples were selected. Frequencies of the three morphotypes are on the lower left. Haplotype frequencies by specific morphotype are on the right. 35 Objective 3 – Describe patterns of genetic variation in cutthroat trout within and among major drainages in the study landscape Genetic differences among drainages were apparent in all analyses, as evidenced by a) average pairwise nucleotide differences within and between drainages (Table 14), b) a non-random distribution of haplotypes among drainages (2 = 232.67; P < 0.00001), and c) an overall pairwise GST of 0.14. The differences in haplotype distribution among drainages can be visualized in Figures 13 and 15. These differences were quantified with pairwise population GST values (Table 16). Two distinct haplotype clades were present in the dataset (Figures 14 and 15). Clade 1 was distributed throughout the study area, but there was a tendency for this group of haplotypes to be more common in Jackson Hole and the Gros Ventre, while clade 2 haplotypes were more common in the Hoback, Snake River Canyon, and Greys (Figures 13 and 15). Based on these observations, drainages were grouped into the two clades; Clade 1 including Jackson Hole and the Gros Ventre, and Clade 2 including the Hoback, Snake River Canyon, and Greys. When these two groups were compared, haplotypes were found to be non-randomly distributed between them (2 = 105.64; P < 0.00001), and genetic differentiation between these groups was detectable (G ST = 0.07218). Genetic diversity differed among drainages (Table 15). The Hoback and Greys drainages had the highest levels of nucleotide diversity ( ; which takes haplotype divergence into account), but Jackson Hole and the Greys had the highest haplotypic diversity (Hd) and the largest number of haplotypes represented. Table 14 Average pairwise genetic distances (and standard errors) between individuals within (along diagonal, shaded) and between geographic groups of cutthroat trout in the Snake River headwaters, Wyoming. Distances within and between groups are expressed as average number of mutational differences (below diagonal, italicized) or average percent of mutational differences (above diagonal). Jackson Hole Gros Ventre Hoback Snake River Canyon Greys All Drainages Jackson Hole Gros Ventre Hoback Snake River Canyon Greys All Drainages 0.002 (0.001) 1.993 (0.85) 2.935 (0.969) 5.432 (1.480) 8.475 (2.413) 4.422 (1.230) n/a 0.003 (0.001) 0.003 (0.001) 3.191 (0.91) 5.289 (1.486) 7.584 (2.221) 4.438 (1.257) n/a 0.006 (0.002) 0.006 (0.002) 0.006 (0.002) 5.651 (1.47) 5.479 (1.551) 5.441 (1.499) n/a 0.009 (0.003) 0.008 (0.002) 0.006 (0.002) 0.003 (0.001) 2.428 (0.68) 6.331 (1.812) n/a 0.005 (0.001) 0.005 (0.001) 0.006 (0.002) 0.007 (0.002) 0.005 (0.001) 5.122 (1.34) n/a n/a 36 n/a n/a n/a n/a 0.005 (0.001) 4.361 (1.19) Table 15 Genetic diversity indices for cutthroat trout in Snake River headwaters drainages. Nucleotide diversity (), haplotype diversity (Hd), and number of haplotypes are presented for each drainage. (SD) Hd (SD) # Haplotypes 0.0021 (0.00013) 0.00337(0.00058) 0.00598(0.00020) 0.00292(0.00110) 0.00542(0.0010) 0.711(0.021) 0.601(0.063) 0.660(0.039) 0.286(0.112) 0.717(0.040) 8 7 4 4 7 Population Jackson Hole Gros Ventre Hoback Snake River Canyon Greys Table 16 Genetic differentiation among cutthroat trout in Snake River headwaters drainages, based on haplotype distributions, characterized using the GST statistic (Nei 1987; Hudson et al. 1992). Jackson Hole Gros Ventre Hoback Snake River Canyon Greys Jackson Hole n/a Gros Ventre 0.05500 n/a Hoback 0.07087 0.05890 n/a Snake River Canyon Greys 0.16493 0.24884 0.10040 n/a 0.05014 0.01183 0.00926 0.14504 n/a All Drainages n/a n/a n/a n/a n/a 37 All Drainages 0.13737 HAPLOTYPES Jackson Hole A n = 93 B C D HAPLOTYPES Gros Ventre n = 62 H ol e H J B Ja ck so n M A C D H HAPLOTYPES Snake River Canyon Gros Vent K re L n = 26 so ck Ja A D n I le Ho L Sn e ak on ny a rC ve i R HAPLOTYPES Hoback n = 40 Ho b ac k A B D J ys Gre HAPLOTYPES Greys n = 57 A B C D E F H Figure 13 Frequencies of haplotypes A-M varied among the five geographic areas within the Snake River headwaters, Wyoming. Four haplotypes were dominant, with two (B and D) occurring throughout the study landscape. Haplotype A was present mainly in the Hoback, Snake River Canyon and Greys. Haplotype C occurs mainly in tributaries in the Teton Mountains within Jackson Hole. 38 YSCT1 SRC02 M L YSC55 J D K 84 H Clade1 C YSC53 B 98 G YSCA1 I Clade 2 A 43 70 F E BRC 70 LHC 74 WSC CRC GBC 100 RXC RBT Figure 14 Dendrogram of haplotypes A-M identified in the Snake River headwaters, Wyoming. Cutthroat trout out groups include: BRC – Bonneville, CRC – Colorado River, GBC – greenback, LHC – Lahontan, SRC02 – finespotted Snake River, WSC – west slope, YSCA1, YSCT1, YSC53 and YSC55 – Yellowstone. Rainbow trout (RBT) and rainbow-cutthroat hybrid (RXC) haplotypes are included in this unrooted neighbor-joining tree. Values at branches are the relative strengths of nodes (percent) assessed by bootstrapping 1,000 times; scale is 5 base pair difference. 39 D J YSC L K C B M H CLADE 1 ? G ? ? BRC E– ? ? ? F I Jackson Hole Gros Ventre =5 Hoback =1 Snake River Canyon ? Greys A CLADE 2 Figure 15 Network of cutthroat trout haplotypes A-M, with frequency of occurrence for each of five geographic areas indicated by symbols. YSC is a single occurrence haplotype from Yellowstone Lake; BRC is Bonneville cutthroat trout. Haplotype network was produced using statistical parsimony. 40 Objective 4 – Detection of Rainbow Trout Introgression A total of 12 fish were captured between 1998 and 2003 that were field identified as either rainbow trout (RBT) or rainbow-cutthroat trout hybrids (RXC; Table 17). Six fish were captured in the Gros Ventre and field identified as RXC. Five of those were captured in the Gros Ventre R, and one fish in Crystal Cr. A single fish was captured in the Hoback River and identified in the field as a RXC. Five fish captured in Fawn Cr, within the Greys River drainage were identified in the field as RBT. The main identifying feature for all of the hybrid fish was either white margins or tips on the pelvic and anal fins. Review of each of the available photographs (one film slide from Fawn Cr was too poor quality and subsequently discarded) supported the field identifications. Tissue from 8 of the 12 fish identified as RBT or RXC were included in our analyses. No photograph or tissue was collected from 3 of the RBT in Fawn Creek due to their being <150 mm TL. The RXC captured in Crystal Creek was not included in this analysis. Genetic differences were observed in 6 of the 8 fish that distinguished them from cutthroat trout. Those 6 fish grouped with RBT as depicted in the neighbor-joining dendrogram (Fig. 14). While four distinct haplotypes were observed from the 6 fish, only one haplotype was used in constructing the tree. The two remaining RXC, both Dhaplotype fish, clearly exhibited white on the pelvic and/or anal fins. Lack of a RXC haplotype in these two fish emphasizes that mtDNA only expresses maternal inheritance. No other instance of an RXC haplotype was observed in the sample set. The thirty samples from the Snake River specifically screened due to concerns of potential hybridization did not exhibit any RXC haplotypes. Sequencing of the mtDNA ND2 gene essentially functioned as a fine-filter screening of all 324 samples from throughout the study landscape. This suggests that hybridization is largely limited to those locales previously suspected of harboring RBT or RXC, the main exception being the fish captured in the Hoback (Figure 16). Also, the RXC captured upstream of Lower Slide Lake in Crystal Cr and the Gros Ventre R were the first documented hybrid trout being present above the lake and indicate a greater range in the Gros Ventre than was previously known (Figure 16). 41 Table 17 Locations and fish metrics for five rainbow trout (RBT) and seven rainbow-cutthroat trout hybrids (RXC) captured in the Snake River headwaters, Wyoming. Drainage/Stream Location (m) Species Length (mm) Weight (gm) Sample ID Crystal Cr1 10,000 RXC 374 495 GV-03-02-344 Gros Ventre R 20,000 RXC 327 350 GV-03-03-284 Gros Ventre R GROS VENTRE RIVER 20,000 RXC 341 445 GV-03-03-287 R1 34,500 RXC 357 490 GV-03-02-273 Gros Ventre R1 48,000 RXC 318 265 GV-03-02-221 Gros Ventre R1 50,000 RXC 378 520 GV-03-01-209 20,000 RXC 373 510 GH-03-01-123 Fawn Cr 750 RBT 220 100 GH-00-07-02 Fawn Cr 1,500 RBT 153 39 GH-00-07-04 Fawn Cr 1,500 RBT 110 16.0 NA2 Fawn Cr 1,500 RBT 105 12.0 NA Fawn Cr 1,500 RBT 94 8.0 NA Gros Ventre HOBACK RIVER Hoback R GREYS RIVER 1 Capture locations upstream of Lower Slide Lake were outside of previous known range in the Gros Ventre River drainage. 2 No photograph or fin clip collected for fish <150 mm TL. 42 Ho le n Ja ck so Gros Ve ntre so ck Ja n le Ho ke na ve Ri an rC n yo Ho b S ac k ys Gre rainbow-cutthroat trout hybrids rainbow trout Figure 16 Presence of rainbow trout or rainbow-cutthroat trout hybrids in Gros Ventre and Greys River drainages were previously known or suspected. The capture of rainbow-cutthroat trout hybrids in the Hoback R, and upstream of Lower Slide Lake in the Gros Ventre were the first documented. 43 DISCUSSION Develop cost-effective, reliable, and repeatable molecular tools that will answer the study questions A set of robust primers were designed and optimized that allowed us to amplify and sequence the most variable region of the ND1-2 gene. Nearly all (>95%) of the approximately 530 samples selected from our study landscape, plus 75 out group samples, amplified using this protocol. The sequences obtained from the 324 cutthroat trout from our study landscape and 65 individuals from the out groups represent 92% of the amplicons attempted being successfully sequenced. Within the entire data set, 110 of the 945 base pairs were variable. Eighteen variable sites defined the 13 different haplotypes within the cutthroat trout from our study landscape (Appendix A Table 21). Numbers of fish bearing a specific haplotype are listed by stream in Appendix A (Table 21). The primer set was similarly successful in amplifying and sequencing the cutthroat trout out groups, rainbow trout, and rainbow-cutthroat trout hybrids. The number of base pair substitutions between cutthroat trout in the Snake River headwaters and the Yellowstone cutthroat trout out group (from Yellowstone Lake and Yellowstone River headwaters upstream of the lake) increased by one to 19, where as there were 59 variable sites among the Colorado River, greenback, Lahontan, and west slope cutthroat trout out groups. The number of base pair substitutions increased to 77 between cutthroat trout in the Snake River headwaters and rainbow trout. Such a high rate of intraspecific variation as was found in the mtDNA ND2 gene of the cutthroat trout (as noted above, 59 variable sites among the five subspecies), is one of three key properties that make mtDNA so favorable to phylogeographic studies. The remaining key properties are maternal transmission, and absence of genetic recombination in this haploid genome (Avise 2000). That higher intraspecific variation in mtDNA is more likely to show differences among populations than single-copy nuclear DNA, arises in part, from the smaller effective population size (one-quarter that of the bisexually inherited diploid nuclear genome; Billington 2003). While the mtDNA sequences were useful in confirming hybridization in morphologically suspect fish, a particular deficiency is the inability to address degree of cutthroat trout introgression with rainbow trout, also a result of the single-locus haploid genome. The nuclear marker system, microsatellites, suggested similar successes. A total of 17 loci from the three primer sets were optimized, and polymorphism was identified at five of the seven loci evaluated. Specifically, four microsatellite loci were found to be robust and polymorphic (6-14 alleles/locus) in our optimization sample set (n=16). Two of the four were OCH loci, and 2 of the loci were obtained from IDFG. One locus suggested to us by the IDFG lab, while variable, was much less polymorphic (3 alleles), and one locus showed no variability within our cutthroat trout samples. These four polymorphic microsatellite loci, and the protocols for their amplification, are available for future studies on fine-scale (geographically and temporally) genetic structuring in 44 cutthroat trout in the Snake River headwaters. The development and optimization of primers is commonly the most time consuming portion of generating microsatellite data, and this work is an important contribution to future research. Microsatellite data could tell us more about introgression with rainbow trout, and barriers to gene flow discussed below because they are nuclear, codominant, and have a high mutation rate (Brown and Epifanio 2003; Gharrett and Zhivotovsky 2003). It is quite likely that very pronounced structuring would be determined due to identification of unique alleles, and fixation of alleles (Gharrett and Zhivotovsky 2003; Shaklee and Currens 2003); identification of diagnostic markers in out groups is also possible. Genetic Differentiation among Morphotypes Three recognized sub-species of cutthroat trout have been described within Wyoming that inhabit tributaries of the Bear River (Bonneville cutthroat, O. c. utah), Colorado/Green River (Colorado cutthroat, O.c. pleuriticus), and the Yellowstone River and Snake River (Yellowstone cutthroat, O. c. bouvieri, YSC). While a fine-spotted morphotype (finespotted Snake River, SRC) has been identified in the Snake River headwaters of northwest Wyoming (Baxter and Simon 1970; Behnke 1992), we were unable to genetically and morphologically differentiate this morphotype from the Yellowstone cutthroat trout. Previous morphological and genetic investigations have examined differences in the fine-spotted and large spotted morphotypes, but have not conclusively differentiated the two types (Loudenslager 1978; Loudenslager and Kitchin 1979; Loudenslager and Gall 1980). The presence of a large number of fish with intermediate spotting patterns within our survey suggests that these fish are clearly not reproductively isolated and that there is considerable overlap in morphology and meristics. Behnke (1992) suggested that variation and overlap in meristic counts and observations of intermediate spotting may indicate continued gene flow and acknowledged that the difference in spotting pattern, and observed intermediate spotting, may result from simultaneous expression of two co-dominant alleles at one locus. More recent analyses by Kruse et al. (1996) of YSC in the Greybull River drainage (Missouri River drainage) of northwest Wyoming showed no consistent difference in counts of seven meristic features of fish sampled from 18 streams. Genetic differentiation among morphotypes was not apparent, either within drainages or when samples were pooled across the entire study area. We could find no recognizable separation of morphotypes within the mitochondrial haplotypes we identified. All spotting patterns were represented in the upper Snake River, as well as each of the major drainages that were sampled. There was no evidence that these morphotypes represented distinct lineages. Previous genetic comparisons of YSC and SRC (Leary et al. 1987, Allendorf and Leary 1988) with allozyme electrophoresis did not discern diagnostic markers at the many loci analyzed. While we were unable to differentiate two distinct morphotypes, the conservation of unique color and spotting patterns may be important for future management. Unique phenotypes and life histories in westslope cutthroat trout, and physiological adaptations 45 in related interior cutthroat are examples of such variation that exists and that should be maintained (Carl & Stelfox 1989; Taylor et al. 2003). Genetic Differentiation among Major Drainages While we were unable to discriminate differences between the finespotted and large spotted morphotype, we were able to detect haplotype frequency differences among the major drainages that we surveyed. The differences we observed indicate two major clades that are loosely represented by a haplotype group associated with the two northern geographic areas (Jackson Hole, Gros Ventre) and a haplotype that occurs predominantly in the three southern geographic areas (Snake River Canyon, Hoback, Greys). These clades are likely to have evolved in response to different hydrogeographic conditions than those that exist today. A large landscape analysis would be necessary to make statements about ancient barriers that may have produced these clades. Caution should be exercised when making conclusions regarding the Snake River Canyon fish because no cutthroat trout from the main Snake River were included in our sample. Haplotypes appear to be unsorted among different drainages, suggesting that there are no differences in lineages among different drainages. There are two possible explanations; first, that the current patterns of isolation have not been in place long enough for lineages to arise and second, that barriers among drainages are not complete, but sufficient to prevent homogenization of haplotype frequencies. Most likely the current situation is a combination of the two. Taylor et al. (2003) found that there was significant genetic divergence of westslope cutthroat populations in the upper Kootenay River and that populations appeared to be demographically independent despite the ability of fish to move freely among some of the drainages. They recommended that these populations be treated as distinct biological units for the purposes of management. There appear to be an anomalous morphotype associated with haplotype C. These fish occur almost exclusively above barriers, and in four canyons within the Teton Mountains, in Jackson Hole. The morphotype exhibits very large (>5 mm), and disperse spots, not observed anywhere else in the study landscape. While we cannot determine the origin of these differences, it is likely a result of isolation of fish with the more ancient haplotype C (Figure 15). Review of the stocking records for the four streams in question (Cascade Cr, Death Canyon, Leigh Canyon, and Paintbrush Canyon) indicates that only Leigh Canyon had a documented introduction, likely of finespotted Snake River cutthroat trout. Although no record exists, it is assumed cutthroat trout have been stocked in Cascade Canyon due to the presence of brook trout above the barrier falls, and the likelihood of stocking Lake Solitude (the headwater source) due to its popularity as a back packing destination and trail access. Thus, it is quite likely that presence of haplotype D in Cascade Cr and haplotypes B and D in Leigh Canyon resulted from introductions of cutthroat trout via stocking. Regardless, the fact that these anomalous fish are extant in Cascade Cr and Leigh Canyon, and are the only morphotype and haplotype present in Death Canyon and Paintbrush Canyon, is indicative of the 46 historical genetic variation present in cutthroat trout within the study landscape. Significant portions of within species diversity may be partitioned between populations above and below barriers (Carlsson and Nilsson 1999; Costello et al. 2003). Their demographic independence may indicate that conservation and recovery plans should take into account the importance of these isolated populations (Taylor et al. 2003). Detection of Rainbow Trout Introgression There is little evidence for the widespread hybridization of cutthroat trout with rainbow trout in the Snake River headwaters of Wyoming. Our data suggests that hybridization is largely limited to those areas that were previously suspected of harboring RBT or hybrids due to rainbow trout stocking. Two notable exceptions are the single hybrid captured in the Hoback River, and several hybrid captured upstream of Lower Slide Lake in the Gros Ventre River drainage. Some caution must be exercised relative to the genetic techniques used in this study. The use of mitochondrial DNA may underestimate the true hybridization extent because of maternal inheritence. Other techniques (e.g., specifically nuclear markers such as allozymes, microsatellites, PINES) may be more appropriate to further examine the full extent of hybridization (Henderson et al. 2000, Hitt et al. 2003). Two fish that were identified in the field as hybrids, but were genetically represented as pure using mtDNA should be screened using a different technique to confirm the initial call. Hybrid identification using the appearance diagnostics was corroborated by genetic analysis in 6 of 8 fish, indicating those diagnostics are useful to quickly screen potential hybrids for genetic analysis. Henderson et al. (2000) found that field techniques using spotting pattern, body color, mandible length, and presence or absence of coloration below the gill covers were effective at screening YSCxRBT hybrids with a misclassification rate of 2%. The addition of these characteristics in future sampling of YSC and SRC to examine the invasion of hybrids could increase reliability in identification of hybrids in the field. There are a number of potential reasons why hybridization may not be widespread in the upper Snake River. Rainbow trout stocking occurrences were limited to discrete areas within the drainage and were not as widespread or repeated as stocking in the river below Palisades Reservoir (Henderson 1998). Reproductive isolation may be important in preventing hybridization between related fish species (Hubbs 1955; Leary et al. 1995). Spatial or temporal separation (Thurow 1988; Huston et al. 1984; Likenes and Graham 1988) during spawning may prevent hybridization in the few streams where introduced rainbow trout and native cutthroat trout coexist. For example, fewer YSCxRBT hybrids were found in tributaries to the Snake River below Palisades Reservoir when compared to the number of hybrids in the mainstem (Henderson et al. 2000). Hybrids that were found in tributaries were typically in the lower portions of tributaries, while pure YSC were found higher in the drainage. Given the limited numbers of hybrids that we found, it is difficult to determine the direction of hybridization within the headwaters tributaries. Follow up studies should be conducted 47 in the areas where hybrids were currently identified to determine whether the proportion of hybrids is changing. One caution that should be noted is that very few of our fish came from the mainstem Snake River. Given our sampling we have little idea of the extent of hybridization that exists in the main river from Jackson Lake to Palisades Reservoir. If patterns of invasion below Palisades Reservoir are any indication, the initial invasion front may be located in the main river and not further up the tributaries. There appears to be no environmental gradient that would retard or stop RBT or YSCxRBT hybrids from occupying portions of the main river or tributaries. This is a concern given that a wild, reproducing RBT population (including RXC) is present and connected (via Palisades Reservoir) in the Salt River drainage (Figure 1). Observations on the spread of hybridization in the Flathead River system indicate that the presence of neighboring populations of hybrids is more important than environmental characteristics, particularly when environmental conditions are favorable for both species (Hitt et al. 2003). Further investigation of the mainstem should be attempted to determine the presence and extent of hybrids to inform future management. Management Recommendations Initiate a landscape level analysis using this sample set and nuclear markers (microsatellites) to understand historic geologic and hydrologic conditions that may explain the patterns of genetic variability observed in this study. While not conclusive, frequency differences among drainages suggest that there should be caution in translocating cutthroat trout among drainages. Initiate mainstem Snake River investigation to better determine the presence, location, and extent of hybridization in the river between Palisades Reservoir and Jackson Lake. 48 REFERENCES CITED Allendorf, F. W., and R. F. Leary. 1988. Conservation and distribution of genetic variation in a polytypic species, the cutthroat trout. Conservation Biology 2:170184. Baxter, G. T., and J. R. Simon. 1970. Wyoming fishes. Wyoming Game and Fish Commission Bulletin 4. Baxter, G. T., and M. D. Stone. 1995. Fishes of Wyoming. Wyoming Game and Fish Department, Cheyenne, WY. Behnke, R. J. 1992. Native trout of western North America. American Fisheries Society Monograph 6. Behnke, R. J. 2002. Trout and salmon of North America. The Free Press. New York. Carl, L.M. and J.D. Stelfox. (1989). A meristic, morphometric and electrophoretic analysis of cutthroat trout Salmo clarki from two mountain lakes in Alberta Canada. Canadian Field Naturalist, 103, 80–84. Carlsson, J. and J. Nilsson. (1999). Effects of geomorphological structures on genetic differentiation among brown trout populations in a northern Boreal river drainage. Transactions of the American Fisheries Society, 130, 36–45. Campbell, M. R., J. Dillon, and M. S. Powell. 2002. Hybridization and introgression in a managed, native population of Yellowstone cutthroat trout: Genetic detection and management implications. Transactions of the American Fisheries Society 131:364-375. Clement, M., D. Posada, and K. A. Crandall. 2000. TCS: a computer program to estimate gene genealogies. Molecular Ecology 9:1657-1660. Condrey, M.J., and P. Bentzen. 1988. Characterization of coastal cutthroat trout (Oncorhynchus clarki clarki) microsatellites and their conservation in other salmonids. Molecular Ecology 7:783-792. Costello AB, Down TE, Pollard SM, Pacas CJ, Taylor EB (2003). The influence of history and contemporary stream hydrology on the evolution of genetic diversity within species: an examination of microsatellite DNA variation in bull trout, Salvelinus confluentus (Pisces: Salmonidae). Evolution, 57, 328–344. 49 Fausch, K. D., C. E. Torgerson, C. V. Baxter, and H. W. Li. 2002. Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. Bioscience 52(6):483-498. Folt, C. L., K. H. Nislow, and M. E. Power. 1998. Implications of temporal and spatial scale for Frissell, C. A. 1997. Ecological principles. Pages 96-115 in J.E. Williams, C.A. Wood, and M.P. Dombeck, editors. Watershed restoration: principles and practices. American Fisheries Society, Bethesda, Maryland. Gresswell, R. E. 1988. Status and management of interior stocks of cutthroat trout. American Fisheries Society Symposium 4. Gresswell, R. E. 1995. Yellowstone cutthroat trout. Pages 36-54 in M.K. Young, technical editor. A conservation assessment for inland cutthroat trout. USDA Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado. General Technical Report RM-GTR-256. Hagenbuck, W. W. 1970. A study of the age and growth of the cutthroat trout from the Snaje River, Teton County, Wyoming. Masters Theses, University of Wyoming, Laramie. Harper, D. D., and A. M. Farag. 2002. Winter habitat utilization and movements by cutthroat trout in the Snake River near Jackson, Wyoming 2000-2001. USDI, U.S. Geological Survey, Columbia Environmental Research Center, Jackson Field Station. Final Report to U.S. Bureau of Reclamation Project 70L31. Harper, D. D., and A. M. Farag. 2004. Winter habitat use by cutthroat trout in the Snake River near Jackson, Wyoming. Transactions of the American Fisheries Society 133:15-25. Hayden, P. S. 1967. Snake River cutthroat trout study. Part I: the reproductive behavior of the Snake River cutthroat trout in three tributary streams in Wyoming. Wyoming Game and Fish Commission and University of Wyoming. Cooperative Research Project 4. Henderson, R.C., J.L. Kershner, and C.A. Toline. 2000. Timing and location of spawning of non-native wild rainbow trout and native cutthroat trout in the South Fork Snake River, Idaho, with implications for hybridization. North American Journal of Fisheries Management 20: 584-596. Hitt, N.P., C.A. Frissell, C.C. Muhlfeld, and F.W. Allendorf. 2003. Spread of hybridization between native westslope cutthroat trout, Oncorhynchus clarki lewisi, and nonnative rainbow trout, Oncorhynchus mykiss. Can. J. Fish. Aquat. Sci. 60: 1440.1451. 50 Hubbs, C. L. 1955. Hybridization between fish species in nature. Systematic Zoology 4:1–20. Hudson, R. R., D. D. Boos, and N. L. Kaplan 1992. A statistical test for detecting geographic subdivision. Molecular Biology and Evolution 9:138-151. Huston, J. E., P. Hamlin, and B. May. 1984. Lake Koocanusa investigations final report. Montana Department of Fish, Wildlife and Parks, Helena. Irwin, D. E. 2002. Phylogeographic breaks without geographic barriers to gene flow. Evolution 56:2383-2394. Jones, K. C. Unpublished microsatellite primers. Genetic Identification Services. http://www.genetic-id-services.com/ Kiefling, J. W. 1972. An analysis of stock densities and harvest of the cutthroat trout of the Snake River, Teton County, Wyoming. Master’s Thesis, University of Wyoming, Laramie. Kiefling, J. W. 1978. Studies on the ecology of the Snake River cutthroat trout. Wyoming Game and Fish Commission, Cheyenne, Wyoming. Fisheries Technical Bulletin 3. Krebs, C.J. 1999. Ecological methodology. 2nd Ed. Addison-Wesley Longman Educational Publishers, Inc. Menlo Park, CA. Kruse, C. G. 1998. Influence of nonnative trout and geomorphology on distributions of indigenous trout in the Yellowstone River drainage of Wyoming. PhD Dissertation, University of Wyoming, Laramie. Kruse, C. G., Hubert, W.A., and F. J. Rahel. 1996. Sources of variation in counts of meristic features of Yellowstone cutthroat trout (Oncorhychus clarki bouvieri). Great Basin Naturalist 56:300-307. Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Briefings in Bioinformatics 5:150-163. Labbe, T. R., and K. D. Fausch. 2000. Dynamics of intermittent stream habitat regulate persistence of a threatened fish at multiple scales. Ecological Applications 10:1774-1791. Leary, R. F., F. W. Allendorf, S. R. Phelps, and K. L. Knudsen. 1987. Genetic divergence and identification of seven cutthroat trout subspecies and rainbow trout. Transactions of the American Fisheries Society 116:580-587. 51 Leary, R. F., F. W. Allendorf, and G. K. Sage. 1995. Hybridization and introgression between introduced and native fish. Pages 91–101 in H. L. Schramm, Jr., and R. G. Piper, editors. Uses and effects of cultured fishes in aquatic ecosystems. American Fisheries Society, Symposium 15, Bethesda, Maryland. Levin, S. A. 1992. The problem of pattern and scale in ecology. Ecology 73:1943-1967. Likenes, G. A., and P. J. Graham. 1988. Westslope cutthroat trout in Montana: life history, status, and management. Pages 53–60 in R. E. Gresswell, editor. Status and management of interior stocks of cutthroat trout. American Fisheries Society, Symposium 4, Bethesda, Maryland. Loudenslager, E. J. 1978. Variation in the genetic structure of populations of two polytypic vertebrates, the cutthroat trout (Salmo clarki) and the deer mouse (Peromyscus maniculatus). Doctor of Philosophy Thesis. University of Wyoming. Loudenslager, E. J., and E. M. Kitchin. 1979. Genetic similarity of two forms of cutthroat trout, Salmo clarki, in Wyoming. Copeia 1979:673-678. Loudenslager, E. J., and G. A. E. Gall. 1980. Geographic patterns of protein variation and subspeciation in cutthroat trout, Salmo clarki. Systematic Zoology 29:27-42. Love, J. D., J. C. Reed, Jr., and K. L. Pierce. 2003. Creation of the Teton Landscape, A Geologic Chronicle of Jackson Hole and the Teton Range. Grand Teton Natural History Association, Moose, WY. May, B. 1996. Yellowstone cutthroat trout Oncorhynchus clarki bouvieri. Pages 11-34 in D.Duff, technical editor. Conservation assessment for inland cutthroat trout: distribution, status and habitat management implications. USDA Forest Service, Intermountain Region, Ogden, Utah. Montgomery, M.R. 1995. Many rivers to cross - of good running water, native trout, and the remains of wilderness. Simon and Schuster. New York, New York. Murphy, T. C. 1974. A study of Snake River cutthroat trout. Master's Thesis, Colorado State University, Fort Collins. Nei, M. 1987. Molecular evolutionary genetics. Columbia University Press, New York. Nelson, J. S., E. J. Crossman, H. Espinosa-Perez, L. T. Findley, C. R. Gilbert, R. N. Lea, and J. D. Williams. 2004. Common and scientific names of fishes from the United States, Canada, and Mexico. American Fisheries Society, Special Publication 29, Bethesda, Maryland. USA. Nelson, R. J., and T. D. Beacham 1999. Isolation and cross species amplification of microsatellite loci useful for study of Pacific salmon. Animal Genetics 30:228-229. 52 Olsen, J. B., P. Bentzen and J. E. Seeb. 1998. Characterization of seven microsatellite loci derived from pink salmon. Molecular Ecology 7:1087-1089. Peacock, M. M., H. M. Neville, and V. S. Kirchoff. 2004. Ten species-specific microsatellite loci for Lahonton cutthroat trout Oncorhynchus clarki henshawi. Molecular Ecology Notes 4:557-559. Quadri, S. U. 1959. Some morphological differences between the subspecies of cutthroat trout, Salmo clarki clarki, and Salmo clarki lewisi, in British Columbia. Journal of the Fisheries Research Board of Canada. 16:903-915. Rexroad, C. E., R. L. Coleman, A. L. Gustafson, W. K. Hershberger, and J. Killefer. 2002. Development of rainbow trout microsatellites markers from repeat enriched libraries. Marine Biotechnology 4(1):12-16. Rieman, B. E., and J. D. McIntyre. 1995. Occurrence of bull trout in naturally fragmented habitat patches of varied size. Transactions of the American Fisheries Society 124:285-296. Rozas, J., J. C. Sanchez-DelBarrio, X. Messeguer, and R. Rozas. 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496-2497. Sakamoto, T., Okamoto, N., Ikeda, Y., Nakamura, Y., and T. Sato. 1994. Dinucleotiderepeat polymorphism in DNA of rainbow trout and its application in fisheries science. Journal of Fish Biology 4:1093-1096. Shiozawa, D. K., and R. N. Williams. 1992. Geographic variation, isolation and evolution of cutthroat trout (Salmo clarki). Final Report to The National Geographic Society. Grant No. 3653-87. Skaala, O., and K. E. Jorstad. 1988. Inheretence of the fine-spotted pigmentation pattern in brown trout. Polish Archives of Hydrobiology 35:295-304. Spruell, P., M. L. Bartron, N. Kanda, and F. W. Allendorf. 2001. Detection of hybrids between bull trout (Salvelinus confluentus) and brook trout (Salvelinus fontinalis) using PCR primers complimentary to interspersed nuclear elements. Copeia 4:1093-1099. Sunnucks, P., and D. F. Hales. 1996. Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae). Molecular Biology and Evolution 13:510-524. Thurow, R. F., C. E. Corsi, and V. K. Moore. 1988. Status, ecology, and management of Yellowstone cutthroat trout in the upper Snake River drainage, 53 Idaho. Pages 25–36 in R. E. Gresswell, editor. Status and management of interior stocks of cutthroat trout. American Fisheries Society, Symposium 4, Bethesda, Maryland. Toline, C. A., T. Seamons, and J. M. Hudson. 1999. Mitochondrial DNA analysis of selected populations of Bonneville, Colorado River, and Yellowstone cutthroat trout. Department of Fisheries and Wildlife, Utah State University, Logan, UT. Trojnar, J. R., and R. J. Behnke. 1974. Management implications of ecological segregation between two introduced populations of cutthroat trout in a small Colorado lake. Transactions of the American Fisheries Society 103:423-430. Varley, J. D., and R. E. Gresswell. 1988. Ecology, status, and management of the Yellowstone cutthroat trout. American Fisheries Society Symposium 4:13-24. Warren, M. L., and B. M. Burr. 1994. Status of freshwater fishes of the United States: overview of an imperiled fauna. Fisheries 19:6-18. Wenburg, J. K., and P. Bentzen. 2001. Genetic and behavioral evidence for restricted gene flow among coastal cutthroat trout populations. Transactions of the American Fisheries Society 130:1049-1069. Wiley, R. W. 1969. An ecological evaluation of the Snake River cutthroat fishery with emphasis on harvest. Masters Thesis, University of Wyoming, Laramie. Williams, J. E., J. E. Johnson, D. A. Hendrickson, S. Contreras-Balderas, J. D. Williams, M. Navarro-Mendoza, D. E. McAllister, and J. E. Deacon. 1989. Fishes of North America endangered, threatened, or of special concern: 1989. Fisheries 14: 220. Wyoming Game and Fish Department. 1997. A stocking history: The Snake River drainage, Wyoming. Wyoming Game and Fish Department, Cheyenne, Wyoming. Wyoming Game and Fish Department. 1999. Yellowstone cutthroat trout: Historical management applications and status and management. Wyoming Game and Fish Department, Cheyenne, Wyoming. Young, M. K. 1995. Conservation assessment for inland cutthroat trout. General Technical Report RM-256. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. Zar, J. H. 1999. Biostatistical analysis. 4th Edition. Prentice-Hall, Upper Saddle River, New Jersey. 54 APPENDIX A Table 18 Summary of the number of records, by river drainage, of individual fish, and the approximate number of those fish that were photographed and/or a caudal fin clip collected. River drainages are listed as they generally occur from north to south. Buffalo Fork Snake River Gros Ventre River Hoback River Greys River Salt River Total Fish Photographs Tissue 58 3,744 2,254 1,131 1,597 364 9,148 58 1,302 1,051 508 905 138 3,962 58 1,230 709 495 881 124 3,497 55 Table 19 Total genomic DNA extractions for several streams1 in each of five geographic areas. The number of extractions per stream varied due to stream length, numbers of fish captured over the minimum size (>150 mm), and number of samples available for each putative cutthroat trout morphotype1. The geographic areas are arranged as they generally occur from north to south. A history of cutthroat trout stocking in each stream is provided. Drainage and Stream Total Type 1 Type 2 Type 3 Totals 1279 123 907 249 Subtotal 9 11 44 19 18 11 3 12 18 25 25 195 Subtotal 12 38 32 20 94 12 11 26 9 23 19 3 5 304 Historically Stocked2 JACKSON HOLE Cascade Cr3 Death Canyon3 Ditch Cr, Middle Fork4 Flat Cr Leigh Canyon3 Mosquito Cr Mosquito Cr, North Fork Pacific Cr4 Paintbrush Canyon3 Snake R Spread Cr, South Fork4 GROS VENTRE Calf Cr Cottonwood Cr Fish Cr, North Fork Fish Cr, South Fork Gros Ventre R Leeds Cr Maverick Cr Moccasin Cr Papoose Cr Park Cr Raspberry Cr Strawberry Cr Tepee Cr 21 3 11 1 11 3 5 0 14 38 20 3 54 4 18 5 8 103 4 8 5 5 0 4 0 4 1 3 3 1 2 40 4 21 19 13 81 7 7 15 5 17 10 0 1 200 4 9 8 2 13 1 4 7 3 3 6 2 2 64 (continued) 56 9 11 23 8 17 N N N Y 1990 Y Y 1970 N Y 1980 N Y 1960 Y 1960 N Y N Y 1990 Y 2000 N N N N N N N Y Table 19 Continued. Drainage and Stream Total HOBACK Bare Cr Bondurant Cr Boulder Cr Bull Cr Cliff Cr Dell Cr Fisherman Cr Fisherman Cr, Middle Fork Fisherman Cr, North Fork Hoback R Jack Cr Kerr Cr Little Granite Cr Mumford Cr Phosphate Cr Rim Draw Shoal Cr Snag Cr West Shoal Cr Willow Cr Subtotal 2 18 22 23 9 26 4 6 12 85 14 3 17 10 6 2 13 1 6 35 314 Subtotal 7 2 11 19 7 16 12 7 12 5 6 104 SNAKE RIVER CANYON Bailey Cr Bailey Cr, West Cabin Cr Coburn Cr Dog Cr Fall Cr Fall Cr, North Fork Fall Cr, South Fork Horse Cr Pine Cr Pritchard Cr Type 1 3 1 4 1 2 1 2 14 1 1 3 3 1 9 (continued) 57 Type 2 2 13 19 14 9 25 4 6 11 82 12 1 17 9 6 2 13 5 35 285 5 2 6 4 6 15 5 2 10 4 3 62 Type 3 2 2 5 1 1 1 1 1 1 Historically Stocked2 N N N N Y Y 1970 Y 1970 N Y 1970 Y 1990 Y 1960 Y Y 1960 N N N Y 1970 N N Y 15 2 4 14 1 1 4 2 1 1 3 33 Y N Y Y Y Y Y Y Y N Y Table 19 Continued. Drainage and Stream GREYS Blind Trail Cr Firebox Cr Flat Cr Greys R Little Greys R Little Greys R, South Fork Lynx Cr Murphy Cr Murphy Cr, North Fork North Corral Cr Three Forks Cr, North5 Sheep Cr Spring Cr Squaw Cr Steer Cr Stewart Cr Unnamed Tributary to Lower Cabin Cr5 Upper Cabin Cr5 White Cr Subtotal Total Type 1 Type 2 Type 3 38 2 14 113 24 24 3 4 4 18 29 11 15 4 25 13 3 34 2 9 110 15 15 1 4 4 18 19 11 15 4 23 12 1 8 9 4 362 1 3 2 5 3 306 5 1 1 34 3 1 3 3 4 1 22 1 2 2 6 6 2 6 1 1 Historically Stocked2 Y N N Y 1990 Y Y N Y Y N Y 1960 Y 1970 Y Y Y N N N N Streams were selected based on the following criteria: 1) Assume no genetic structure associated with spotting pattern; 2) No history of stocking, to extent possible; 3) Connectivity both within and among river drainages; 4) Streams stratified across the 5 geographic areas; 5) Streams spatially representative within each major river drainage; 6) Maximize age-class or size-groups within each stream; 7) Samples selected from throughout occupied length of stream; 8) Select up to 30 samples per stream for analysis; and 9) In longest streams, segregate sample populations according to stream segments. 10) Spotting pattern morphotypes include: Type 1=large-sparse spots; Type 2=fine-dense spots; and Type 3=intermediate to types 1 and 2. 2 Streams with record of stocking any form of cutthroat trout (WGFD 1997). Last stocking after 1960 is indicated by decade. 3 CUT samples from above confirmed natural barriers to upstream fish movement. 4 Additional tributaries to stream are available with fish exhibiting large-sparse spotting morphotype. 5 Samples from above road culvert identified as barrier to upstream movement of at least one lifestage of cutthroat trout. 58 Table 20 Lists the streams and samples, by geographic area1, selected for sequencing the mtDNA ND2 gene region. Contiguous sequences (~1,100 bp) of n=324 samples were completed. Drainage and Stream TOTAL Total Type 1 Type 2 Type 3 543 91 256 196 9 9 20 11 18 11 17 55 23 0 0 11 0 0 3 0 0 14 0 0 0 5 1 4 0 50 3 9 9 9 6 17 4 17 5 6 173 28 63 82 12 17 49 21 7 9 15 3 5 4 5 0 4 1 3 3 1 2 4 7 38 10 3 6 6 0 1 4 5 11 7 3 0 6 2 2 138 23 75 40 8 6 14 3 40 5 3 4 1 2 1 1 1 2 2 3 7 2 27 3 1 2 2 5 0 12 1 0 79 12 45 22 JACKSON HOLE Cascade Cr2 Death Canyon2 Ditch Cr, Middle Fork3 Flat Cr Leigh Canyon2 Pacific Cr3 Paintbrush Canyon2 Snake R Spread Cr, South Fork3 SUBTOTAL GROS VENTRE Calf Cr Fish Cr, North Fork Gros Ventre R Moccasin Cr Papoose Cr Park Cr Raspberry Cr Strawberry Cr Tepee Cr SUBTOTAL HOBACK Bondurant Cr Boulder Cr Bull Cr Dell Cr Hoback R Jack Cr Kerr Cr SUBTOTAL (continued) 59 Table 20 Continued. Drainage and Stream Total Type 1 Type 2 Type 3 7 13 6 7 3 1 1 2 2 1 3 2 2 2 1 3 10 2 3 1 36 7 10 19 9 8 48 14 4 4 19 3 3 3 3 1 0 4 5 3 31 6 2 3 9 1 2 14 5 1 1 6 5 6 1 3 2 2 2 1 117 21 63 33 SNAKE RIVER CANYON Cabin Cr Coburn Cr Fall Cr, North Fork Fall Cr, South Fork Horse Cr SUBTOTAL GREYS Blind Trail Cr Flat Cr Greys R Little Greys R Steer Cr Stewart Cr Three Forks Cr, North4 Unnamed Tributary, Lower Cabin Cr4 Upper Cabin Cr4 SUBTOTAL 1 Streams were selected based on the following criteria: 1) Ensure variation in spotting patterns within each stream was documented by surveys throughout the occupied length of a stream; 2) There was no history of stocking, or at least recent stocking, in each stream to the extent possible; 3) Connectivity existed among all streams selected, both within and between the geographic areas; 4) Minimize spatial clustering of samples within a stream, to the extent possible, by selecting samples from throughout the occupied length of each stream; 5) Minimize spatial clustering of streams, to extent possible, by selecting streams from throughout each geographic area; 6) Ensure that streams were stratified across the five geographic areas; 7) Ensure that streams were stratified within each of the five geographic areas; 8) Include samples from each of the available age classes or size groups within each stream; 9) Include a minimum of n=30 fish from each geographic area or river drainage, where possible, that exhibit the large-sparse spotting pattern (i.e., YSC); 10) Segregate fish into three distinct spot pattern morphotypes: Type 1 – large-sparse, Type 2 – fine-dense, and Type 3 – intermediate to 1 and 2; 11) Morphotypes present must be confirmed based on photographic records from surveys; and 12) Samples to be included in the analysis should be only from those streams where the largesparse morphotype was observed. 2 CUT samples from above natural barriers to upstream fish movement. 3 Additional tributaries available with fish exhibiting large-sparse spotting morphotype. 4 Samples from above road culvert identified as barrier to upstream movement of at least one lifestage of cutthroat trout. 60 Table 21 Number of cutthroat trout1 of the haplotypes A-M, per stream, within five geographic areas in the Snake River study area. The Snake River is split into two geographic areas, Jackson Hole and Snake River Canyon. The geographic areas are arranged as they generally occur from north to south. Haplotypes Drainage and Stream Total JACKSON HOLE Cascade Cr2 Death Canyon2 Ditch Cr, Middle Fork Flat Cr Leigh Canyon2 Pacific Cr Paintbrush Canyon2 Snake R Spread Cr, South Fork Subtotal GROS VENTRE Calf Cr Fish Cr, North Fork Gros Ventre R Moccasin Cr Papoose Cr Park Cr Raspberry Cr Strawberry Cr Tepee Cr Subtotal A B C D E F G H I J K L M Total 64 41 50 132 2 1 1 19 1 8 1 3 1 324 6 9 4 2 1 1 2 3 1 15 1 22 3 1 5 9 3 14 2 1 48 1 1 4 8 6 1 1 5 7 6 5 25 3 53 0 7 6 21 4 1 8 7 3 5 62 1 5 0 0 1 6 0 4 3 7 6 5 10 4 2 2 3 5 37 1 9 9 11 9 18 10 14 52 7 139 1 0 0 1 2 1 2 2 5 0 0 (continued) 61 0 7 0 0 Table 21 Continued. Haplotypes Drainage and Stream HOBACK Bondurant Cr Boulder Cr Bull Cr Dell Cr Hoback R Jack Cr Kerr Cr A B C Subtotal 2 18 SNAKE RIVER CANYON Cabin Cr Coburn Cr Fall Cr, North Fork Fall Cr, South Fork Horse Cr Subtotal 1 8 5 7 1 22 E F G H I J K L M Total 0 2 2 11 2 16 4 3 40 0 3 9 5 7 2 26 2 1 1 11 4 D 4 3 7 0 2 7 1 1 14 1 0 0 0 0 0 1 0 1 0 1 1 0 0 1 2 0 0 (continued) 62 0 0 1 0 0 1 Table 21 Continued. Haplotypes Drainage and Stream GREYS Blind Trail Cr Flat Cr Greys R Little Greys R Steer Cr Three Forks Cr, North3 Unnamed Tributary to Lower Cabin Cr3 Upper Cabin Cr3 Subtotal A 1 5 3 2 1 1 2 15 B C D E F G H I J K L M 5 4 1 1 1 6 11 1 3 1 6 5 16 13 4 8 2 1 2 1 2 6 1 26 2 1 1 0 6 Total 0 0 0 0 0 3 2 57 Samples were selected by stream based on the following criteria: 1) Ensure variation in spotting patterns within each stream was documented by surveys throughout the occupied length of a stream; 2) No history of stocking, or at least recent stocking, in each stream to the extent possible; 3) Connectivity existed among all streams selected, both within and between the geographic areas; 4) Minimize spatial clustering of samples within a stream, to the extent possible, by selecting samples from throughout the occupied length of each stream; 5) Minimize spatial clustering of streams, to extent possible, by selecting streams from throughout each geographic area; 6) Ensure that streams were stratified across the five geographic areas; 7) Ensure that streams were stratified within each of the five geographic areas; 8) Include samples from each of the available age classes or size groups within each stream; 9) Include a minimum of n=30 fish from each geographic area or river drainage, where possible, that exhibit the large-sparse spotting pattern (i.e., YSC); 10) Segregate fish into three distinct spot pattern morphotypes: (1) large-sparse, (2) fine-dense, and (3) intermediate to 1 and 2; 11) Morphotypes present must be confirmed based on photographic records from stream surveys; and 12) Samples to be included in the analysis should be only from those streams where the large-sparse morphotype was observed. 2 Samples from above confirmed natural barriers to upstream fish movement. 3 Samples from above road culvert identified as barrier to upstream movement of at least one life-stage of cutthroat trout. 63 APPENDIX B Fish Photograph Library: JPEG files are the complete set of photographs associated with tissue samples. Each individual fish photograph has a unique identifier, pic_num, on the fish sheets in the database library (see below) is used as the file name. Database Library: separate EXCEL files for each river drainage with complete data set from presence-absence surveys. One additional file is metadata for survey data. Cutthroat trout sample location and extraction information: the file ext_samp_data_060105.xls contains individual sheets by river drainage that includes each sample identified for extraction, as well as a summary table. Also contains individual sheets for each river drainage that include each sample identified for amplification and use in mtDNA sequencing, as well as a summary table. Out group sample sources and extraction information: the file gen_samp_outgroup_053105.xls contains individual sheets for each out group. Each sheet provides source and individual sample data when available. Mitotype data generated from mitochondrial DNA sequences: the file mitotype _data_053105.xls is the raw data generated from the unique mtDNA sequences. 64