Moles and Relationship
advertisement

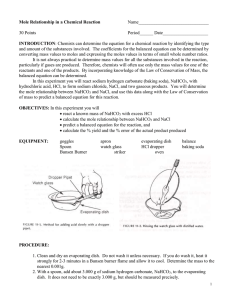
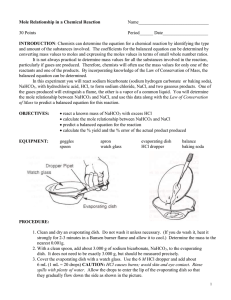
Aim: To find the mole and the mass relationships. Procedure: 1. Flame dry a clean evaporating dish for about 5 minutes and allow the dish to cool. 2. Find the combined mass of the evaporating dish plus a watch glass. 3. Leaving the watch glass and evaporating dish on the balance, move the riders to measure an additional 2.50 g. Using a microspatula, add sodium hydrogen carbonate to the evaporating dish until the scale balances. 4. Place the watch glass on top of the evaporating dish and place the dish on the ring stand. 5. Obtain about 5mL of 6M hydrochloric acid in a clean test tube. Using a dropper pipet, slowly add HCl to the NaHCO3 in the evaporating dish. Continue adding acid until the reaction stops. 6. Using the burner, gently heat the evaporating dish until all the liquid is gone. All the dish to cool and find the combined mass of the watch glass, evaporating dish and NaCl. Data: (a) evaporating dish + watch glass : 37.7 g (b) evaporating dish + watch glass + NaHCO3 : 41.35g (c) evaporating dish + watch glass + NaCl : 40.0g Calculations: 1. The mass of NaHCO3 reactant, (b-a) : 2. The mass of the NaCl product, (c-a) : 41.35g – 37.7g = 3.65g 40.0g – 37.7g = 2.30g Questions and Answers: 1. ·According to the balanced equation for the reaction used in this experiment, what is the ratio of moles of NaHCO3 reacted to moles of NaCl produced? 0.043 mol to 0.039 mol ratio. 2. ·How many moles of NaHCO3 are reacted in this experiment? 0.043 mole. ·How many moles of NaCl are produced? 0.039mole. ·What is the ratio of moles NaHCO3 reacted to moles NaCl produced? 0.043 mol to 0.039 mol ratio. 3. ·Using the balanced equation, calculate the mass of NaCl you would expect to get when 2.50 g of NaHCO3 are reacted with HCl. - In 2.5 g of NaHCO3, there are 0.03 moles of NaHCO3. Therefore in a 1 to 1 ratio, there would be 1.74g of NaCl produced. 4. If the masses of all but one of the substances that take part in a chemical reaction are known, explain why it is possible to determine the unknown mass by subtraction. It is possible to determine the unknown mass by subtraction because the total number of atoms of each element on each side of the equation should be balanced due to the Law of Conservation of Mass and all the needed information is given to you. 5. In the chemical reaction CaCO3 CaO + CO2, if 40.0g of CaCO3 are decomposed: (a) How many grams of CaO are produced? In a 40g of CaCO3, 40% of calcium (16g), 12% of carbon (4.8g), and 16 percent of oxygen3 (19.2g).Since they ask for one calcium and one oxygen, the formula to get the mass would be Ca+O or 16g+6.4g. Thus making there have 22.4 g of CaO produced after the reaction. (b) How many grams of CO2 produced? In a 40g of CaCO3, 40% of calcium (16g), 12% of carbon (4.8g), and 16 percent of oxygen 3 (19.2g).Since they ask for one carbon and two oxygen, the formula to get the mass would be C+ O2 or 4.8g+2(6.4g). Thus making there have 17.6 g of CO2 produced. 6. In the reaction N2 + 3H2 2NH3, if 20.0 grams of hydrogen react: (a) How many grams of ammonia are produced? In a 20g of 3H2, there are 3.34 moles of 3H2. There are 48g of 2NH3 produced. (b) How many grams of nitrogen react? If there is 20 grams of 3H2 reacting then to find the grams of nitrogen reacting you simply subtract the grams of 3H2 from the grams of 2NH3 and you would get an answer of 28 grams of nitrogen