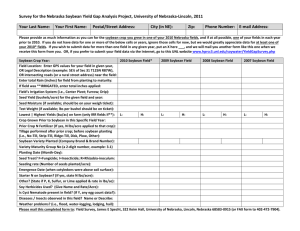
Soybean CV127 - Department of Agriculture
advertisement

Determination of the Safety of BASF’s BPS-CV127-9 Soybean (Herbicide Tolerant) For Direct Use as Food or Feed or for Processing Food and Feed Safety The product dossier BPS-CV127-9 Soybean was reviewed for safety and nutritional differences compared with the conventional soybean. The review was focused on any new or altered expression trait and changes in composition and nutritional content or value relative to the conventional soybean. After thorough evaluation on the safety assessment, the following conclusions were made: BPS-CV127-9 Soybean is as safe as its conventional counterpart taking into its dietary source and nutritional impact of any changes in nutritional value. CV127 soybean is safe to humans, animals and as nutritious as conventional soybean. A biosafety permit for soybean CV127-9 and all progenies derived from crosses of the product with any conventionally soybean containing approved-biotech events for direct use was issued to BASF Philippines, Inc. on October 29, 2010. The validity of the permit was only five years. This approval is for use as food and feed or for processing of soybean CV127-9 in the Philippines. Food and feed use of soybean CV127-9 and its by-products is therefore authorized as of Oct 29, 2010. The biosafety permit (No. 10-051) for direct use stated that “soybean CV127-9 and all progenies from crosses of this product except when stacked with other biotech traits has undergone satisfactory assessment and found to be as safe as conventional soybean and can be a substitute for its traditional counterpart as food and feed or for processing” I. Brief Identification of the Genetically Modified Organism (Living Modified Organism) Designation: BPS-CV127-9 Applicant: BASF Philippines, Inc. 11/F Hanjinphil Corporation Building 1128 University Parkway North Bonifacio, Global City, Taguig, Metro Manila, Philippines Plant Species: Corn : Soybean (Glycine max.) Parent Material : Imidazolinone-tolerant soybean developed by Plant Science (BPS) Center of Origin: Brazil and Argentina Toxic Factors/Allergen(s): Trypsin inhibitors, lectins, urease, stachyose, raffinose and phytic acid. Trait Description: Imidazolinone herbicide tolerance/weed resistance) Trait Introduction Method: Biolistics method BASF phytoestrogens, tolerant (herbicide Donor Organism: Arabidopsis thaliana, source of gene csr1-2 with its native promoter which is a small flowering plant that is widely used as a model organism in plant biology. Pathogenicity: Arabidopis thaliana, the donor organism of csr1-2, is not a known human or animal pathogen and is not known to cause allergic reactions in humans. There is no evidence that A.t. is pathogenic to humans and it is not known to produce toxins or allergens. Proposed Use: For direct use as food, feed and for processing II. Background Information BASF Philippines, Inc. has developed a new genetically modified soybean that is tolerant to the imidazolinone class of herbicides. The imidazolinone herbicides control a wide spectrum of grass and broadleaf weeds and possess high biological efficacy at low application rates. The herbicide tolerant BPS-CV127 soybean (also referred to as CV127) are derived from a single transformation event and was developed to address weed control challenges to soybean growers. Introduction of CV127 soybean varieties will offer soybean growers an additional tool for controlling problem weeds as well as an important option for weed resistance management. The imidazolinone-tolerant soybean exhibits similar herbicide tolerance properties as various imidazolinonetolerant crops produced. On July 8, 2010, BASF Philippines, Inc. submitted an application to the Bureau of Plant Industry requesting for biosafety permit under A.O. #8 for soybean which has been genetically modified for weed resistance. BASF Philippines, Inc. has provided information on the safe history of use of the crop, the source of the donor gene, the molecular characterization of CV127 soybean, the stability of the inserted genetic elements, characterization and expression levels of AHAS proteins produced in the CV127 soybean plant, lack of any allergenicity or toxicity characteristics associated with AHAS protein and CV127 soybean, as well as the nutrient composition of the soybean grain, forage and grain processed fractions, and overall food and feed safety of CV127 soybean plants. Relevant scientific publications were also supplied. CV127-9 Soybean has been evaluated according to BPI‘s safety assessment by concerned agencies: Bureau of Animal Industry (BAI), Bureau of Plant Industry (BPI), Bureau of Agriculture Fisheries and Product Standards (BAFPS) and a Scientific Technical Review Panel (STRP). The process involved an intensive analysis of the nature of the genetic modification with a consideration of general safety issues, toxicological, nutritional and environmental issues associated with the modified soybeans. The petitioner/applicant published the said application on two (2) widely circulated newspapers (Malaya Business Insight and The Daily Tribune) on May 20, 2010 for public comment/review. BPI received positive comments on the petition supporting the application for direct use as food and feed or for processing of CV127-9 Soybean during the 30-day comment period. Review of results of evaluation by the BPI Biotech Core Team completed the approval process. III. Description of Novel Protein (Introduced Traits) The csr1-2 gene that has been inserted into the genome of soybean CV127 is derived from Arabidopsis thaliana which is a member of the mustard (Brassicaceae) family, which includes cultivated species such as cabbage and radish. Arabidopis thaliana is a small flowering plant which is widely used as a model organism in plant biology. CV127 soybeans were produced by introduction of the iminidazolinone-tolerant acetohydroxyacid synthase large subunit gene csr1-2 from Arabidopsis thaliana into the soybean plant genome. The csr1-2 encodes an acetohydroxyacid synthase large subunit (AHASL) protein that is tolerant to imidazolinone herbicide due to a single nucleotide mutation that results in a single amino acid substitution in which the seine residue at position 653 of the protein is known to prevent the binding of imidazolinone herbicides and thereby to result in tolerance to these herbicides. Safety of the Expressed Proteins Arabidopsis thaliana has been cultivated widely as a model plant for genetic and biochemical research without any associated safety concerns and the plant is closely related to plant species in the mustard family that have a history of safe human consumption. The csr1-2 gene encodes the AtAHAS protein that confers tolerance to the imidazolinone herbicides. Biochemical characterization of the AtAHAS protein expressed in CV127 soybean showed that the AtAHAS protein is homologous to other AHAS proteins present in crop plants and has characteristics typical of most dietary proteins with a history of safe use in food and feed products and lacks any characteristics associated with known toxic or allergenic proteins. The Arabidopsis AHASL (AtAHAS) is a member of the class of AHAS proteins found ubiquitously in plants. The AHASL enzyme catalyzes the first step in the biosynthesis of branched-chain amino acids, valine, leucine, and isoleucine. The herbicide tolerance in CV127 soybean will allow growers to treat the soybean crop with imidazolinone herbicides for weed control without causing injury to the soybean plant at normal field application rates. The safety of the AtAHAS protein for human consumption was assessed by several different approaches. Bioinformatic searches of databases of all known proteins, including protein toxins as well as of known allergenic proteins were conducted and it was confirmed that the AtAHAS protein expressed in CV127 soybean does not share immunologically relevant amino acid sequence segments or structure with known allergens or have sequence homology to known protein toxins. Therefore, the AtAHAS protein expressed in CV127 soybean tissues does not pose any attributed known protein food allergens, is not toxic to mammals, and therefore presents no risks for human or animal consumption IV. Nutritional Composition (Compositional Analysis) Studies demonstrated that the mean and range values for proximate analyses for moisture, ash, protein , crude fat, dietary fiber, carbohydrate and calorie content, amino acids, fatty acids and minerals (potassium, magnesium, calcium, iron and phosphorus); vitamins (trocopherols) showed comparable ranges with commercial soybean. The nutritional equivalence of CV127 soybean to conventional soybean was confirmed in several feeding studies with broiler chickens and birds. The studies showed that CV127-9 and the commercial variety are substantially equivalent in comparison. Thus CV127-9 can be used in food and feed formulations and 100% substitute for commercial soybean. V. Anti-Nutritional Factors Although soybean does not produce any known toxic compounds, it does contain antinutrients with very low amount and are below the thresholds considered to raise a food safety concern. These include trypsin inhibitors, lectins, urease, phytoestrogens, stachyose, raffinose and phytic acid. However, the amount of anti-nutrients present in CV127 soybean fell within the range found in conventional standard varieties. VI. Regulatory Decision After viewing the scientific data and information relevant to the application of BASF Philippines, Inc. it is concluded that CV127-9 Soybean and all progenies from crosses of this product except when stacked with other biotech traits for direct use is as safe and substantially equivalent to its unmodified counterpart and is therefore approved for direct use as food, feed and for processing. BASF shall duly inform the public of this approval by the way of publishing in any one (1) of the top three (3) leading newspapers in the country that imports of this product is covered by conditions for approval as provided in Department of Agriculture Memorandum Circular No. 8, Series of 2003. .