Structures of Some Food Dyes-2
advertisement

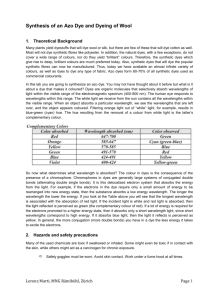
Structures of Some Food Dyes What About the Structure Gives the Color? As you look at each of the structures of the dyes included here, you will see something that they have in common. Each structure has conjugated double bonds. That is, there is a series of alternating single and double bonds with these structures having either 9 or 10 bonds in the "line". Since they are conjugated (the electrons are delocalized in the line of bonds), there are not really alternating single and double bonds but the pi system extends throughout the entire conjugated system. In such a system of this length, the difference in energy between the ground state and the first excited state falls in the visible region of the spectrum. The groups attached to such a conjugated system affect the energies and thus the position within the visible spectrum. That is, wavelengths of light are modified by the electron donating or accepting properties of the groups attached to the conjugated system. Red 40, Yellow 5, and Yellow 6 are called azo dyes because they contain a N=N grouping which is called an azo group. Blue 1 and Blue 2 contain nitrogen atoms but they are widely separated. These dyes are water soluble because they are salts. They contain sulfonic acid groups which are a part of a sulfuric acid molecule bonded to a carbon. These sulfonic acid groups are strong acids which have been neutralized to give the water soluble form of the dyes. What About the Names? These dyes have a variety of names which can make it rather confusing. A table is given listing some of the more common names for the dyes considered here. F D & C Number Red 40 Yellow 5 Name allura tartrazine Yellow 6 sunset yellow FCF Blue 1 brilliant blue FCF Blue 2 indigo carmine indigotine Color Name food red 17 food yellow 4 acid yellow 23 food yellow 3 acid yellow 6 food blue 2 acid blue 9 food blue 1 The numbers don't match. The numbers for the blue dyes are reversed. FD&C Blue 1 is food blue 2 and FD&C Blue 2 is food blue 1. What Happens to the Dye I Eat? If you eat a lot of red M&Ms or drink a lot of red Kool-Aid, why isn't your urine pink? Some of these dyes do pass through the digestive track unchanged but most are metabolized. The molecules are broken down into simpler molecules which are not colored. Some dyes are banned for use in food. Even those which are allowed can cause problems for a small number of people. It isn't the dye causing the problems but the metabolites. This situation is not uncommon. My wife is not allergic to quinine (a component of certain soft drinks primarily used in the preparation of drinks called Tom Collins. But after a couple of hours she breaks out in a rash. She is allergic to a metabolite of quinine so the allergic reaction doesn't show up until the quinine is being metabolized. More information about possible health problems from dyes can be found on the web but read most of it with caution. There are lot of people who put things out there without a whole lot of fact or research behind it. The Structure of FD&C Red 40 Structure of FD&C Yellow 5 Structure of FD&C Yellow 6 Structure of FD&C Blue 1 Structure of FD&C Blue 2 Web Links http://www.red40.com/pages/chemistry.html This site has a brief discussion of dyes but the most impressive aspect of this site are the structures. There are ball and stick models of Red 40, Yellow 5 and Yellow 6 plus a banned dye, Sudan 1. If you roll the cursor over the structure of Red 40 it turns into Yellow 6. This allows you to see the differences in the structures of the two dyes and how similar they really are. The same is true for the other structures. Wikipedia has pages on the various dyes. There is little chemistry given except for indigo which is not a food dye. If you want to know all the various names for a particular dye, you will find it. Beware of any information about health hazards – the only references given are to newspaper articles, not to genuine scientific studies. Even when Wikipedia sounds good, it may be incorrect.