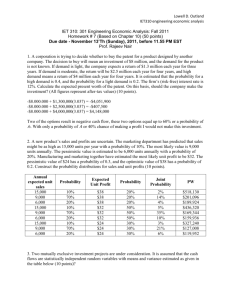
Table 1: Comparison of demographic and medical characteristics of
advertisement

Table 1: Comparison of demographic and medical characteristics of the two study groups in the ITT population Diclectin Placebo P-Value (n-131) (n=125) 0.48 Ethnicity: 53 (40.5%) 56 (44.8 %) Hispanic or Latino 78 (59.5%) 69 (55.2 %) Not Hispanic or Latino Race: 0.59 Asian 2 (1.5%) 1 (0.8%) Black or African American 49 (37.4%) 48 (38.4 %) White or Caucasian 80 (61.1%) 73 (58.4 %) Unknown 3 (2.4%) Previous Pregnancy 101 (77.1%) 94 (75.2 %) 0.64 Smoking during pregnancy 17 (13.0%) 16 (12.8 %) 0.97 Maternal Age (yr) 25.96 25.05.7 0.23 Weight (kg) (lbs) 74.1022.30 163.3549.17 75.9122.19 167.3448.91 0.50 0.50 BMI (kg/m²): Underweight Normal Overweight Obese BMI (kg/m²) MeanSD Median Gestational age at start of NVP (weeks) MeanSD 0.42 5 (3.8%) 39 (29.8%) 31 (23.7%) 55 (42.0%) 0.95 28.777.60 27.97 29.6711.20 26.83 0.90 5.51.8 Gestational age at enrollment (weeks) MeanSD 9.32.0 PUQE score at Enrollment MeanSD Median 4 (3.2%) 38 (30.4%) 40 (32.0%) 42 (33.6%) 5.41.7 0.75 9.31.8 0.44 9.02.1 9.0 8.82.1 8.0 Global Assessment of Well Being MeanSD 5.02.3 5.42.2 Median 5.0 5.0 Table 2 Overall Summary of Tolerability/Adverse Events for ITT-S Subjects ______________________________________________________________________________ ____Treatment Group____ Diclectin (N=131) Placebo (N=127) P-value1 Measure of Tolerability ______________________________________________________________________________ Number of Subjects with at least one treatment- 74 (56.5%) 65 (51.2%) 0.393 emergent AE Number of Subjects with a serious treatment4 (3.1%) 4 (3.1%) emergent AE Number of Subjects with at least one Related AE 40 (30.5%) 32 (25.2%) Number of Subjects discontinuing study drug due to AE 6 (4.6%) 4 (3.1%) Number of deaths 0 0 Overall treatment0emergent AEs Number of Subjects with at least one Mild AE 62 (47.3%) 59 (46.5%) Number of Subjects with at least one Moderate AE 5 (3.8%) 1 (0.8%) Number of Subjects with at least one Severe AE 7 (5.3%) 5 (3.9%) Number of Subjects with Unrelated AE 34 (26.0%) 33 (26.0%) Number of Subjects with at least one Possibly 24 (18.3%) 23 (18.1%) Related AE Number of Subjects with at least one Probably 13 (9.9%) 8 (6.3%) Related AE Number of Subjects with at least on Definitely 3 (2.3% 1 (0.8%) Related AE 1.0002 0.339 0.7492 _ 0.221 0.2152 0.711 0.570 0.714 0.388 0.6232 ______________________________________________________________________________ 1 The p-value for comparing Treatment groups uses Chi-square test method. P-value is calculated using Fisher’s exact test method. Related category includes Possible, Probable, and Definite relationships. Unrelated category includes unlikely and not related. Subjects reporting more than one AE will only be counted under the strongest relationship and/or severity. 2 Table 3 Treatment Emergent Adverse Events (TEAEs) in the Study for ITT-S Subjects ______________________________________________________________________________ System Organ Class (SOC) Preferred Term P-value1 Diclectin (N=131) Treatment Group___ Placebo (N=127) ______________________________________________________________________________ # of Subjects with at least one TEAE 74 (56.5%) 65 (51.2%) 0.39 Cardiac disorders Palpitations Eye disorders Dry eye Gastrointestinal disorders Constipation Dry mouth Haematemesis Feeling jittery Laboratory Investigations Alanine aminotransferase increased Aspartate aminotransferase increased Blood albumin decreased Blood amylase increased Blood chloride decreased Blood creatinine increased Blood lactate dehydrogenase increased Blood sodium decreased Blood triglycerides increased Gamma-glutamyltransferase increased Heart rate increased Platelet count decreased Nervous system disorders Dizziness Headache Loss of consciousness Poor quality sleep Somnolence Syncope Fatigue 1 (0.8%) 1 (0.8%) 1 (0.8%) 1 (0.8%) 23 (17.6%) 2 (1.5%) 4 (3.1%) 0 1 (0.8%) 7 (5.3%) 0 0 1 (0.8%) 2 (1.5%) 0 1 (0.8%) 1 (0.8%) 0 1 (0.8%) 1 (0.8%) 0 1 (0.8%) 42 (32.1%) 8 (6.1%) 17 (13.0%) 0 1 (0.8%) 19 (14.5%) 1 (0.8%) 9 (6.9%) 1 (0.8%) 1 (0.8%) 0 0 22 (17.3%) 2 (1.6%) 1 (0.8%) 1 (0.8%) 0 6 (4.7%) 1 (0.8%) 1 (0.8%) 0.4922 0 2 (1.6%) 1 (0.8%) 1 (0.8%) 0 1 (0.8%) 0 1 (0.8%) 1 (0.8%) 0 37 (29.1%) 8 (6.3%) 20 (15.7%) 1 (0.8%) 0 15 (11.8%) 1 (0.8%) 8 (6.3%) 1.0002 1.0002 1.0002 1.0002 0.960 1.0002 0.3702 0.4922 1.0002 0.820 0.4922 1.0002 1.0002 0.4922 1.0002 1.0002 0.4922 1.0002 1.0002 0.4922 1.0002 0.610 0.949 0.526 0.4922 1.0002 0.523 1.0002 0.853 ______________________________________________________________________________ 1 The p-value for comparing Treatment groups uses Chi-square test method. P-value is calculated using Fisher’s exact test method. At each level of summarization (SOC/preferred term), subjects reporting more than one AE will only be counted once. 2 Table 4 Most Frequently Occurring Treatment Emergent Adverse Events (TEAEs) in the Study for ITT-S Subjects ______________________________________________________________________________ System Organ Class (SOC) Diclectin Treatment Group___ Placebo Preferred Term P-value1 (N=131) (N=127) ______________________________________________________________________________ # of Subjects with at least one TEAE Gastrointestinal disorders Abdominal pain General disorders and administration site Conditions Fatigue Musculoskeletal and connective tissue Disorders Back pain Nervous system disorders Dizziness Headache Somnolence 74 (56.5%) 23 (17.6%) 5 (3.8%) 13 (9.9%) 65 (51.2%) 22 (17.3%) 8 (6.3%) 12 (9.4%) 0.393 0.960 0.362 0.897 9 (6.9%) 11 (8.4%) 8 (6.3%) 4 (3.1%) 0.949 0.072 7 (5.3%) 42 (32.1%) 8 (6.1%) 17 (13.0%) 19 (14.5%) 4 (3.1%) 37 (29.1%) 8 (6.3%) 20 (15.7%) 15 (11.8%) 0.383 0.610 0.949 0.526 0.523 ______________________________________________________________________________ 1 The p-value for comparing Treatment groups uses Chi-square test method. P-value is calculated using Fisher’s exact test method. TEAEs that are considered most frequently occurring include the events (in preferred terms) reported by at least 5% of subjects in any of the treatment groups. At each level of summarization (SOC/preferred term), subjects reporting more than one AE will only be counted once. 2 Table 5 Treatment Emergent Adverse Events (TEAEs) with Respect to Relationship to Study DrugRelated vs. Unrelated for ITT-S Subjects ______________________________________________________________________________ Treatment Group__________ Diclectin Placebo (N=131) (N=127) __________________________________ System Organ Class (SOC) Preferred Term Related Unrelated Related Unrelated ______________________________________________________________________________ # of Subjects with at least one TEAE in the study Cardiac disorders Palpitations Eye disorders Dry eye Gastrointestinal disorders Abdominal pain 40 (30.5%) 34 (26.0%) 32 (25.2%) 1 (0.8%) 1 (0.8%) 0 0 8 (6.1%) 1 (0.8%) 0 0 1 (0.8%) 1 (0.8%) 15 (11.5%) 4 (3.1%) 0 0 0 0 8 (6.3%) 3 (2.4%) 33 (26.0%) 1 (0.8%) 1 (0.8%) 0 0 14 (11.0%) 5 (3.9%) Abdominal pain upper Constipation Diarrhea Dry mouth Dyspepsia Flatulence Salivary hypersecretion General disorders and administration Feeling jittery Nervous system disorders Dizziness Headache Loss of consciousness Poor quality sleep Somnolence Syncope Fatigue 0 1 (0.8%) 2 (1.5%) 4 (3.1%) 1 (0.8%) 0 0 7 (5.3%) 1 (0.8%) 33 (25.2%) 6 (4.6%) 8 (6.1%) 0 0 19 (14.5%) 1 (0.8%) 6 (4.6%) 3 (2.3%) 1 (0.8%) 2 (1.5%) 0 4 (3.1%) 0 0 6 (4.6%) 0 9 (6.9%) 2 (1.5%) 9 (6.9%) 0 1 (0.8%) 0 0 3 (2.3%) 2 (1.6%) 1 (0.8%) 1 (0.8%) 1 (0.8%) 1 (0.8%) 0 0 6 (4.7%) 0 24 (18.9%) 5 (3.9%) 8 (6.3%) 0 0 15 (11.8%) 0 5 (3.9%) 3 (2.4%) 1 (0.8%) 1 (0.8%) 0 1 (0.8%) 1 (0.8%) 1 (0.8%) 6 (4.7%) 0 13 (10.2%) 3 (2.4%) 12 (9.4%) 1 (0.8%) 0 0 1 (0.8%) 3 (2.4%) ______________________________________________________________________________ Related category includes Possible, Probable, and Definite relationships. Unrelated category includes unlikely and not related. At each level of summarization (SOC/preferred term), subjects reporting more than on AE will only be counted once under the strongest relationship.