Specific targeting of leukaemia stem cells in HTLV
advertisement
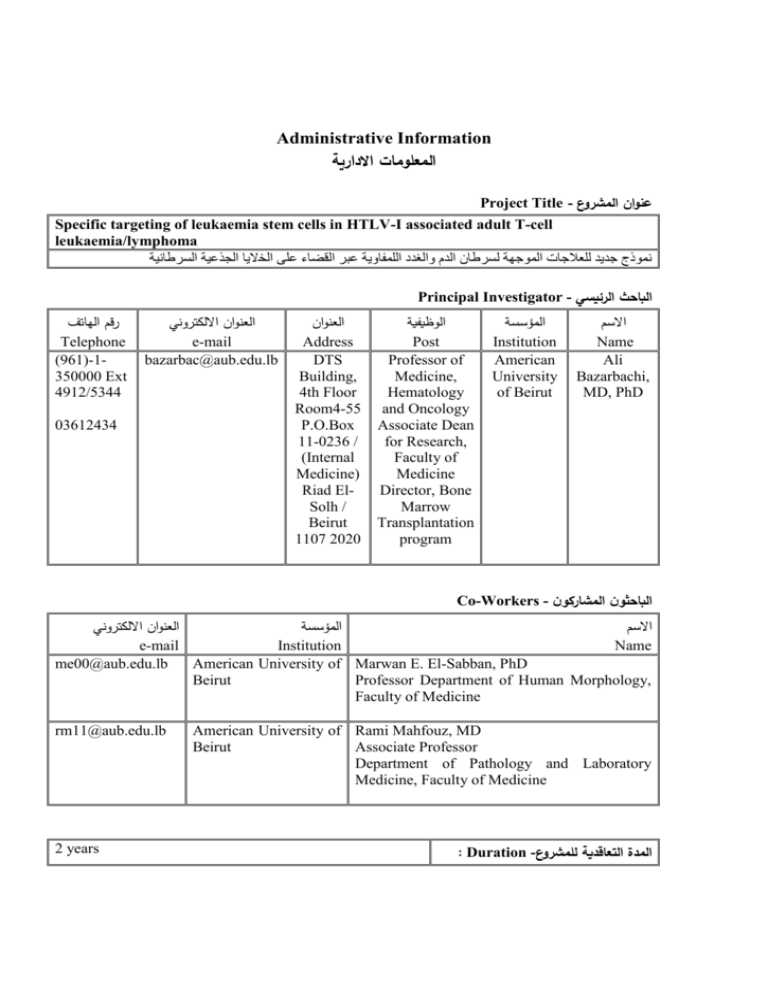
Administrative Information المعلومات االدارية Project Title - عنوان المشروع Specific targeting of leukaemia stem cells in HTLV-I associated adult T-cell leukaemia/lymphoma نموذج جديد للعالجات الموجهة لسرطان الدم والغدد اللمفاوية ﻋﺑﺭ الﻗﺿاﺀ ﻋلﻰ الﺨﻻﻴا الجذﻋﻴة السرطانﻴة Principal Investigator - الباحث الرئيسي رقم الهاتف Telephone (961)-1350000 Ext 4912/5344 العنوان االلكتروني e-mail bazarbac@aub.edu.lb 03612434 العنوان Address DTS Building, 4th Floor Room4-55 P.O.Box 11-0236 / (Internal Medicine) Riad ElSolh / Beirut 1107 2020 الوظيفية Post Professor of Medicine, Hematology and Oncology Associate Dean for Research, Faculty of Medicine Director, Bone Marrow Transplantation program المؤسسة Institution American University of Beirut االسم Name Ali Bazarbachi, MD, PhD Co-Workers - الباحثون المشاركون العنوان االلكتروني المؤسسة االسم e-mail Institution Name me00@aub.edu.lb American University of Marwan E. El-Sabban, PhD Beirut Professor Department of Human Morphology, Faculty of Medicine rm11@aub.edu.lb 2 years American University of Rami Mahfouz, MD Beirut Associate Professor Department of Pathology and Laboratory Medicine, Faculty of Medicine : Duration -المدة التعاقدية للمشروع Scientific Information العلمية المعلومات ّ Objectives - الهدف The area of research activity of this proposal is in the targeted therapy of leukemia by agents other than DNA-damaging chemotherapeutic agents. This targeted therapy is associated with a higher efficacy and lower toxicity. In prior studies, we have discovered that combined IFN/As was extremely potent in inducing apoptosis in ATL cells in culture. Focusing on the cellular and molecular parameters involved in this treatment revealed that both programmed cell death and inhibition of proliferation are involved in vitro. Indeed, this treatment targets two critical pathways for the survival of the leukemic cells: specific proteasome-mediated degradation of the viral oncoprotein Tax and inhibition of an important pathway in cellular proliferation, NF-κB. We also deciphered the molecular and cellular effects of the proteasome inhibitor PS-341. We have also discovered that posttranslational modification of the principal oncoprotein Tax, by ubiquitin and Sumo, plays a critical role in the activation of the NF-κB pathway in ATL. In the in vivo Tax transgenic mouse model, we demonstrated that the combination of As and IFN, synergistically cures murine ATL most likely through leukaemia initiating cell (LIC) exhaustion, rather than immediate growth inhibition or apoptosis. Indeed, the disease initially thrives under therapy and only vanishes 3 weeks later. A brief As/IFN treatment that does not change ATL cell abundance or viability, results in a dramatic loss of the ability of ATL cells to transplant in secondary recipients. Strikingly, the addition of the proteasome inhibitor (PS-341) to As/IFN protects LIC and surprisingly results in a dramatic increase in circulating malignant cells. The general aim of this proposal was the specific targeting of leukaemia stem cells in HTLV-I associated adult T-cell leukaemia/lymphoma. To reach this main goal, we had 4 specific aims: Specific aim 1: Leukemia Initiating Cells. Specific aim 2: Tax post translational modifications (Ubiquitylation/Sumoylation). Specific aim 3: Global gene expression profile in ATL SCID mice using DNA microarrays. Specific aim 4: Tax driven leukemogenesis. Achievements -أالنجازات المحققة 1- We showed that the combination of arsenic trioxide and interferon-alpha eradicates leukemia initiating activity in TAX-driven murine adult T cell leukemia. These results were published in Journal of Experimental Medicine (Impact Factor 14.5; Appendix 1, attached). (El Hajj H, El-Sabban M, Hasegawa H, Zaatari G, Ablain J, Saab S, Janin A, Mahfouz R, Nasr R, Kfoury Y, Nicot C, Hermine O, Hall W, de Thé H and Bazarbachi A. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J Exp. Med. 2010; 207(13):2785-92).Summary: Adult T cell leukemia (ATL) is one of the rare human cancers initiated by a transforming retrovirus, HTLV-I. After many years of controversy, it is now accepted that the viral transactivator protein Tax plays a critical role in initiating the leukemic process, because Tax transgenics develop a disease with striking ATL features. Long-term prognosis of ATL patients remains extremely poor. We have previously shown that the combination of arsenic trioxide and interferon alpha is highly synergistic to induce cell cycle arrest and apoptosis of ATL cells (Bazarbachi et al. 1999). We also showed that this combination works through reversal of NF-kB activation and proteasome degradation of Tax (El-Sabban et al. 2000; Nasr et al. 2003). Here we capitalized on our previous in vitro results to explore the effect of the combination of arsenic trioxide and interferon alpha in vivo on murine ATL and human ATL. To investigate the molecular mechanism of therapeutic action in vivo, we used Tax transgenics that develop a disease with striking ATL features. We demonstrate that the combination of arsenic trioxide and interferon-α cures Tax-driven ATLs. Unexpectedly, this combination therapy abrogated initial leukemia engraftment into secondary recipients, while the primary tumor bulk still grew in the primary hosts, only to ultimately abate later on. This loss of initial transplantability requires proteasome function. Our demonstration that this drug combination targeting Tax stability, abrogates tumor cell immortality, but not short-term growth, foretells a favorable long-term efficiency of this regimen in patients. 2- We demonstrated the role of Tax post-translational modifications in Tax localization and NF-kB activation. The results were published in Blood (Impact Factor 10.5; Appendix 2 attached). (Kfoury Y, Setterblad N, El-Sabban M, Zamborlini A, Dassouki Z, El Hajj H, Hermine O, Pique C, de Thé H, Saïb A, and Bazarbachi A. Tax ubiquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood 2011 Jan 6;117(1):190-9). Summary: The HTLV-I oncoprotein Tax is critical for T cell transformation, acting mainly through NEMO binding and subsequent NF-kB activation. Tax displays a dual nuclear and cytoplasmic localization and is subjected to post-translational modifications. We previously showed that whereas Tax ubiquitylation is essential for targeting and binding the IkappaB kinase signalosome at the centrosome, SUMOylated Tax accumulates in Rel-A enriched Tax nuclear bodies (NBs) and both modifications lead to complete transcriptional activation (Nasr et al. 2006; Kfoury et al. 2008). By using a photoconvertible fluorophore coupled with live video confocal microscopy, we show that the same Tax molecule shuttles between Tax NBs and the centrosome depending on its post-translational modification. Ubiquitylation targets Tax to SUMO/Rel-A enriched NBs and recruits NEMO to these Tax bodies and mediates its subsequent SUMOylation. In agreement with this observation, we show that the SUMO ligase Ubc9 is also recruited to these Tax NBs. Finally, ubiquitylation and SUMOylation of Tax allow NEMO targeting to the centrosome. Altogether, we are proposing a model where ubiquitylation and SUMOylation regulate both Tax and NEMO shuttling between the nuclear and cytoplasmic compartments, assigning a new enzymatic role to Tax NBs. 3- We also performed a worldwide meta-analysis showing that first line antiviral therapy significantly improves survival of patients with acute, chronic and smoldering ATL. These results were published in Journal of Clinical Oncology (Impact Factor 17.5; Appendix 3 attached). (Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, Gessain A, Harrington W, Panelatti G, Hermine O. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010 Sep 20;28(27):4177-83). Summary: The poor prognosis of ATL is due to chemotherapy resistance. Multiple small studies using zidovudine (AZT) and interferon-α (IFN) showed response in ATL patients. However, the global impact of this innovative antiviral treatment strategy on long-term survival of ATL patients in routine practice remains undetermined. We realized a worldwide meta-analysis on the use of AZT/IFN treatment. Charts of 254 ATL patients treated from 1994 to 2008 were individually reviewed. Five year overall survival rates were 20% for 77 patients who received chemotherapy alone, 46% for 75 patients who received first line antiviral therapy alone (p=0.012), and 12% for 55 patients who received chemotherapy followed by antiviral therapy. Response rate to first line antiviral therapy was 66%, including 35% complete remission (CR). When overall survival analysis was performed by ATL subtype, patients with acute, chronic, and smoldering ATL significantly benefited from first line antiviral therapy, whereas patients with ATL lymphoma had a better outcome with first line chemotherapy. Achievement of CR with first line antiviral therapy resulted in prolonged survival of more than 10 years in 82% of the acute ATL subgroup. Finally, first line antiviral therapy in chronic and smoldering ATL resulted in 100% overall survival at 10 years. Multivariate analysis using Cox regression showed that first line antiviral therapy significantly improves overall survival of ATL patients. In conclusion, these results confirm that treatment of ATL using AZT and IFN results in a high response and CR rates particularly in acute, chronic and smoldering ATL, but not in lymphoma, resulting in impressive prolonged survival and hence should be considered as gold standard first line therapy. Perspectives - آفاق البحث Specific aims 3 and 4 are yet to be investigated Publications & Communications - المنشورات والمساهمات في المؤتمرات 1- Bazarbachi et al., Journal of Clinical Oncology 2010. Meta-Analysis on the Use of Zidovudine and Interferon-Alfa in Adult T-Cell Leukemia/Lymphoma Showing Improved Survival in the Leukemic Subtypes 2- Kfoury et al., Blood 2010. Tax uniquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. 3- El Hajj et al., Journal of Experimental Medicine 2010. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia Abstract - موجز عن نتائج البحث The area of research activity of this proposal is in the targeted therapy of leukemia by agents other than DNA-damaging chemotherapeutic agents. This targeted therapy is associated with a higher efficacy and lower toxicity. In prior studies, we have discovered that combined IFN/As was extremely potent in inducing apoptosis in ATL cells in culture. Focusing on the cellular and molecular parameters involved in this treatment revealed that both programmed cell death and inhibition of proliferation are involved in vitro. Indeed, this treatment targets two critical pathways for the survival of the leukemic cells: specific proteasome-mediated degradation of the viral oncoprotein Tax and inhibition of an important pathway in cellular proliferation, NF-κB. We also deciphered the molecular and cellular effects of the proteasome inhibitor PS-341. We have also discovered that post-translational modification of the principal oncoprotein Tax, by ubiquitin and Sumo, plays a critical role in the activation of the NF-κB pathway in ATL. In the in vivo Tax transgenic mouse model, we demonstrated that the combination of As and IFN, synergistically cures murine ATL most likely through leukaemia initiating cell (LIC) exhaustion, rather than immediate growth inhibition or apoptosis. Indeed, the disease initially thrives under therapy and only vanishes 3 weeks later. A brief As/IFN treatment that does not change ATL cell abundance or viability, results in a dramatic loss of the ability of ATL cells to transplant in secondary recipients. Strikingly, the addition of the proteasome inhibitor (PS-341) to As/IFN protects LIC and surprisingly results in a dramatic increase in circulating malignant cells. (Attached publications)