TESTING THE EFFECTIVENESS OF ANTACIDS
advertisement

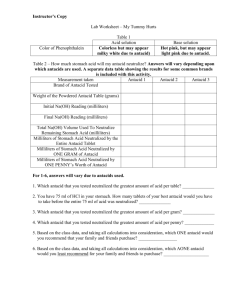
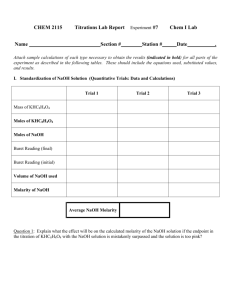
Battle of the antacids Did you know that your stomach produces between 1 L and 2 L of gastric juice daily? The main constituent in this juice is hydrochloric acid, and the pH of a normal stomach is about 1.5 - acidic enough to dissolve magnesium metal! The purpose of this very acidic medium is to digest food. But the stomach itself is made of muscle, so why does it not digest itself? The stomach copes with normal levels of acidity because it completely replaces its lining every few days. However, if the concentrations of acid in the stomach are too high, problems can occur. Pain, swelling, inflammation, and bleeding in the stomach’s lining can cause a condition commonly called “acid indigestion”. One solution to the problem is to take a medication that contains an antacid - a mild base that can neutralize acid. The typical bases found in common antacids include sodium bicarbonate (Alka-Seltzer), calcium carbonate (Tums & Rolaids), and magnesium hydroxide (Maalox). Liquid Universal Indicator pH Color 4.0 Red 5.0 orange red 5.5 orange 6.0 yellow orange 6.5 yellow 7.0 yellow green 7.5 green 8.0 dark green 8.5 blue green 9.0 blue 9.5 violet 10.0 reddish violet PREDICTION: Which over-the-counter antacid is most effective in neutralizing acid? MATERIALS safety goggles large test tube & stopper 100 mL graduated cylinder balance mortar & pestle liquid universal indicator dilute hydrochloric acid (HCl) 3 different antacid tablets Microdroppers PROCEDURE 1. Put on your safety goggles and then gather your equipment. 2. Use a mortar and pestle to crush your antacid tablet. Measure and record the mass (g) of the crushed tablet. 3. Put the antacid sample in the test tube. Add 10 mL of distilled water and 10 drops of the universal liquid indicator. Stopper the test tube and then shake it until the antacid is dissolved. What is the pH of the sample (see chart above)? 4. Remove the stopper and add 1 drop of HCl. Stopper the test tube and shake it. Continue adding 1 drop of HCl, stoppering and shaking until the color of the liquid indicates a pH of 7.0 5. Record the number of drops of HCl added. 6. Rinse your glassware with water and then repeat steps 2 to 5 for the other antacid sample. 7. When you are finished clean up your lab station, return all your equipment, and wash your hands. 8. Calculate and record the neutralizing ability of each antacid (#drops ÷ mass). DATA TABLE: Antacid Mass of 1 tablet Starting pH # of drops of HCl the antacid was able to neutralize # drops/ mass ANALYSIS 1. This experiment required you to be very accurate. What are 2 major sources of error that could have affected your results? Explain each. 2. Based on your data, which antacid neutralized the stomach acid the best? Explain your answer. 3. What is an antacid? Would you want an antacid to dissolve in your stomach instantly or over a period of time? Explain. 4. Fish muscle contains bases that give cooked fish their “fishy” odor. Suggest why a squirt of lemon juice often makes this odor disappear. 5. One way to treat a lake polluted with acid rain is to add calcium hydroxide to it. How does this help? Why is this only a short-term “fix” to the acid rain problem for the lake?