FULL ARTICLE - Kerala Medical Journal
advertisement

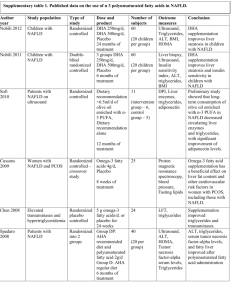
CORRELATION OF SERUM URIC ACID LEVELS TO THE PREVALENCE AND SEVERITY OF NON ALCOHOLIC FATTY LIVER DISEASE: A CROSS SECTIONAL STUDY IN SOUTH INDIAN MALES Dr.T.P. Antony, Dr. Iwin David, Dr. Alex Thomas, Dr. Sojan George K, Dr. Robert Ambookan, Dr. Sachin Menon Department of Internal Medicine, Amala Institute of Medical Sciences, Amala Nagar, Trichur District, Kerala, India. __________________________________________________________________________________ ABSTRACT: BACK GROUND: Increased uric acid is associated with the metabolic syndrome and insulin resistance. Non-alcoholic fatty liver disease (NAFLD) is the commonest manifestation of insulin resistance. Very few studies compare the association between serum uric acid levels and NAFLD. OBJECTIVES: To assess the co-relation of serum uric acid levels with the prevalence and severity of NAFLD in apparently healthy south Indian males. METHODS: Among the individuals attending the master health check up program at a tertiary care centre in central Kerala, 216 apparently healthy male subjects without other risk factors were enrolled in the study. Serum uric acid levels were measured in a 12 hour fasting venous sample, along with fasting blood sugar, lipid profile, liver function tests, calcium, phosphate, urea and creatinine. Serum uric acid levels were categorized into the following quartiles: < 3.0, 3.1-5.0, 5.0-6.5 and > 6.5 mg/dL. Hepatic steatosis was diagnosed and graded based on abdominal ultrasonographic findings by hyperechogenicity of liver in comparison to kidney, difference of echogenicity between the liver and diaphragm, and visibility of vascular structures. The data was analysed using the Chi square test and logistic regression analysis. RESULTS: Of the 216 subjects included in the study, the prevalence of NAFLD was found to be 54.17%. Among these 85.47% had grade 1 and 14.53% had grade 2 hepatic steatosis. The prevalence of NAFLD in the various quartiles were 20% (0 - 3.0), 31.3% (3.1 - 5.0), 50% (5.1 6.5) and 92.5% (6.5 - 10) which was statistically significant (p<0.005). The proportion of patients with higher grades of steatosis was more in the higher quartiles. Grade 2 NAFLD was seen only among those with serum uric acid levels > 5mg/dl, of which 70.60% had uric acid levels > 6.5 mg/dL. Among those with grade 1 steatosis, 79% of subjects had uric acid levels above 5mg/dl with 37% having levels >6.5 mg/dL. CONCLUSION: Serum uric acid levels were found to have a positive correlation with the prevalence and severity of non alcoholic fatty liver disease even within the physiological limits. KEYWORDS: NAFLD; Uric acid 1 .INTRODUCTION: Non alcoholic fatty liver disease (NAFLD) comprises a spectrum of hepatic pathologies that resemble alcoholic liver disease in subjects without excessive alcohol consumption1. The spectrum of NAFLD includes simple hepatic steatosis, which over time can progress to NASH, with the subsequent development of fibrosis and cirrhosis. It is now known that many patients with hitherto identified “cryptogenic” cirrhosis in fact have liver disease on the basis of NASH, with the resolution of the steatosis once patients become catabolic due to cirrhosis. Increasingly, patients are being identified with cryptogenic cirrhosis who have most likely had NASH for decades. These patients can develop liver failure and require liver transplantation, and some patients can progress to the development of hepatocellular cancer2.Uric acid is an independent risk factor for various metabolic abnormalities, most notably the metabolic syndrome 3 , 4 which has insulin resistance as the basic underlying defect. Hyperuricemia often precedes the development of hyperinsulinemia 5, 6, obesity7, and diabetes 8, 9 in the metabolic syndrome. It has been hypothesized that uric acid can play a role in the development of non-alcoholic fatty liver disease. 10,11. In our study we examined the correlation between the levels of serum uric acid and fatty liver grades detected sonologicaly , which was a practical and reliable method for detecting fatty liver12,13 in an asymptomatic population. 2. METHODS: 2.1 STUDY SUBJECTS: After getting institutional ethics committee approval and detailed informed consent, a total of 590 patients of age between 15 and 60 yrs attending the master health check up clinic in a tertiary centre in central Kerala between 1.2.10 and 31.7.10 were interviewed. Since gender related differences play a major role in the incidence of NAFLD, only male subjects were included in the study. Subjects with history of excessive alcohol intake (> 140 gms/week); past history of liver diseases, cholecystectomy, gout, malignancies or other systemic illness; positive viral hepatitis serology; serum creatinine > 2 mg/dL and those who have taken hepatotoxic medications in the last 3 yrs were excluded from the study. The exclusion procedure involved surveys on alcohol intake, self reported past medical history and laboratory tests. Alcohol intake was assessed through two open questions (1) How often did you have a drink containing alcohol per week in the past 6 months? (2) How many glasses did you have on a typical day when you were drinking in the past 6 months? For the 2nd question one glass of alcoholic beverage was calculated based on the type of beverage consumed (beer - 4%, wine - 12% and foreign liquor - 40 %). From the answers to the above questions subjects were excluded after calculating their daily alcohol intake. After exclusions, of the total 590 patients, 216 apparently healthy south Indian males were included in the study. 2.2 MEASUREMENTS: Because liver biopsy in apparently healthy subjects is not ethical and the sample size was large, a diagnosis of fatty liver was based on abdominal ultrasonography. Liver with any degree of hepatic steatosis was considered fatty in the present study. Hhepatic steatosis was diagnosed by abdominal B mode ultrasonography using a GE Voluson 730 pro 3.5-5 Mega Hertz curvilinear transducer carried out by a single experienced radiologist who did not have access to the subjects clinical or lab results. Hepatic steatosis was assessed semi quantitatively, and graded on the basis of abnormally intense, high level echoes arising from the hepatic parenchyma, liver-kidney differences in echo amplitude, echo penetration into the deep portion of the liver and clarity of the liver blood vessel structure 15, 16. Images were captured in a standard fashion with the patient in the supine position with the right arm raised above the head. An ultrasonographic diagnosis of fatty liver was defined as the presence of a diffuse increase of fine echoes in the liver parenchyma compared with the kidney or spleen parenchyma. Based on the observed findings they were divided into 3 grades of fatty infiltration 14. Grade 1 slight diffuse increase in fine echoes in the hepatic parenchyma with normal visualization of the diaphragm and intra hepatic vessel borders. Grade 2 moderate diffuse increase in fine echoes in the hepatic parenchyma with slightly impaired visualization of the intra hepatic vessels and diaphragm. Grade 3 marked increase in fine echoes in the hepatic parenchyma with poor or no visualization of the intra hepatic vessels borders posterior portion of right lobe of liver and diaphragm After a 12 hour overnight fast, blood samples were collected from all subjects from the ante cubital vein. Fasting blood sugar (FBS), total cholesterol, triglycerides, low-density lipoprotein(LDL) and high-density lipoprotein (HDL) cholesterol, phosphate, calcium, albumin, bilirubin, aspartate aminotransferase (AST), Alanine aminotransferase (ALT), urea, serum creatinine and uric acid were measured by enzymatic methods using the dry chemistry method with the Johnson & Johnson Vitros 5,1 FS automated chemistry analyzer. The following criteria were used to define normal levels of the components assessed: FBS < 100mg/dl, PO4 < 4.5mg/dl, calcium < 10.2mg/dl, albumin < 5 g/dl ,bilirubin < 1mg/dl ,SGOT < 35 u/l ,SGPT < 35 u/l ,ALP < 128 u/l ,Total cholesterol < 200mg/dl ,triglycerides < 150mg/dl ,HDL < 60mg/dl ,LDL < 180mg/dl ,Urea < 40mg/dl and creatinine < 1.1mg/dl. Normal uric acid levels were reported as 3 – 6.5 mg/dl. For the purpose of research, patients were divided on the basis of their uric acid levels into various quartiles (<3), (3-5), (5-6.5) and (>6.5). 2.3 STATISTICAL ANALYSIS: The distribution of hepatic steatosis and hyperuricemia were compared using a chi-square test. Logistic regression analysis was used to analyse the independent relationships of fatty liver and the measured variables. Statistical significance was indicated at p value < 0.05. All statistical analyses were performed using SPSS software version 10.0 KO. VARIABLES MEAN + STANDARD ERROR SIGNIFICANCE(P) FBS 100.94+2.10 0.001* PHOSPHATE 3.89+0.03 0.781 CALCIUM 9.33+0.02 0.176 ALBUMIN 4.39+0.02 0.122 TOTAL BILIRUBIN 0.61+0.02 0.841 SGOT 37.20+1.07 0.137 SGPT 51.11+2.33 0.067 ALP 79.13+1.41 0.565 TOTAL CHOLESTEROL 201.42+2.61 0.005* TRIGLYCERIDES 145.60+5.51 0.355 HDL 44.32+0.72 0.120 LDL 129.91+2.50 0.051 24.71+0.51 0.240 CREATININE 0.93+0.01 0.082 URIC ACID 5.63+0.08 0.001* UREA * P<0.05 3. RESULTS: The mean age of our patients was 48 .5 +/- 8 yrs. The mean BMI of our patients 25.2 kg/ m2 . Of the 216 subjects included in the study, the prevalence of NAFLD was found to be 54.17%. Among these 85.47% had grade 1 and 14.53% had grade 2 hepatic steatosis. Among the various parameters assessed, serum uric acid levels, fasting blood sugar and total cholesterol levels were found to have an association with the presence of hepatic steatosis which was statistically significant (p value < 0.05). The prevalence of NAFLD in the various quartiles were 20% (0 - 3.0), 31.3% (3.1 - 5.0), 50% (5.1 - 6.5) and 92.5% (6.5 - 10). The difference was statistically significant (p<0.005). The prevalence of hepatic steatosis was greatest (92.5%) in subjects whose uric acid levels were above the upper limit of normal range (> 6.5 mg/dl). Among those with uric acid levels above 5mg/dl, the prevalence of steatosis was 50%. The prevalence of steatosis was 31.3 % and 20 % in the lower quartiles (3.1 – 5.0 mg/dl and 0 – 3.0 mg/dl respectively). In patients with uric acid levels of 0 - 3.0mg/dl, 80% had no steatosis while 20 % had grade 1 NAFLD. In those with uric acid levels 3.1 - 5.0 mg/dl, 31.2% had grade 1 NAFLD. 50.0% of patients with uric acid levels in the 3rd quartile (5.1 - 6.5 mg/dl) had steatosis with 44.75 having grade 1 and 5.3 % grade 2 NAFLD. In the last quartile (6.5 – 10 mg/dl) 7.5had no steatosis, 69.8 5 had grade 1 NAFLD and 22.7 % had grade 2 NAFLD. Grade 2 NAFLD was seen only among those with serum uric acid levels > 5mg/dl, of which 70.60% had uric acid levels > 6.5 mg/dL. Among those with grade 1 steatosis, 79% of subjects had uric acid levels above 5mg/dl with 37% having levels >6.5 mg/dL. 80 70 FATTY LIVER 60 50 GRADE 0 40 GRADE 1 30 GRADE 2 20 10 0 <3 3.1-5 5.1-6.5 >6.5 URIC ACID LEVELS Fig 1. Correlation of NAFLD in various uric acid quartiles Fig 2: Proportion of NAFLD in various quartiles of Uric acid FATTY LIVER URIC ACID 0 1 2 TOTAL 0-3 4 1 0 5 Row % 80 20 0 100 3.1-5 44 20 0 64 Row % 68.8 31.3 0 100 5.1-6.5 47 42 5 94 Row % 50 44.7 5.3 100 6.5-10 4 37 12 53 Row % 7.5 69.8 22.6 100 TOTAL 99 100 17 216 Row % 45.8 46.3 7.9 100 4. DISCUSSION: NAFLD is now recognized worldwide as an important cause of chronic liver disease. The prevalence of NAFLD in this study was 54.17 %. This is more than the prevalence reported in various other studies in the male population. The difference can be explained because of the higher BMI of our patients and the study population being not fully representative of the general population. Further population based studies will be required to estimate the exact prevalence of NAFLD in central Kerala. In logistic regression analysis, we observed a significant and independent relationship between uric acid levels and the presence of hepatic steatosis. Our results are in agreement with previous studies. Serum uric acid was independently associated with biopsy-proven hepatic steatosis in a study of 1915 Chinese patients aged 12–80 years with chronic hepatitis B infection17. Li et al.18 also reported similar results in a study of 8925 (6802 men) apparently healthy Chinese. The HERAS study by Yong Jae Li et al reported similar results in a prospective study conducted in 3768 Koreans19. However to prove a role in causation further elaborate studies are required. This study also reveals a correlation between the severity of non alcoholic fatty liver disease and uric acid levels even within the normal range. Moreover grades above grade 1 were always associated with uric acid levels above 5mg/dl. These findings are in concordance with our understanding regarding the association between serum uric acid concentrations and insulin resistance. Thus, the significant association between serum uric acid and NAFLD suggest that insulin resistance is a possible mechanism linking uric acid with NAFLD. Despite the significant relationship between uric acid and NAFLD in the present study, it cannot be concluded whether serum uric acid concentration is a risk factor actively involved in the development of NAFLD. Because this study is of cross-sectional design, causal relationship cannot be determined, warranting prospective studies. Our study has several limitations. First, because study subjects were volunteers visiting master health checkup clinic in a single centre and belonged to an affluent, healthier group compared to community-based cohorts, the study population may not be representative of the general population. Therefore, this study may have been affected by selection bias. Second, only males were included in the study. Different factors could influence the prevalence of NAFLD in females hence the data cannot be generalized to the whole population. Third, the effect of other potential confounding variables on uric acid concentration, e.g., diet pattern, a history of medications with hyperuricemic activity was not considered. Fourth, central obesity is more likely to be associated with metabolic syndrome and NAFLD. However waist circumference was not measured in this study. Finally, although liver biopsy is the gold standard for the diagnosis of fatty liver, it was not performed in the present study. However, liver biopsy is an invasive and impractical procedure in the standard clinical setting. Ultrasonography is a non-invasive and widely available method for qualitative assessment of fatty infiltration. Thus, ultrasonography is the preferred modality for mass screening for hepatic steatosis and has reasonable sensitivity (up to 94%) and specificity (up to 84%) 20. CONFLICTS OF INTEREST: None. ACKNOWLEDGMENT: To Mrs. Jini M.B. our statistician. REFERENCES: [1]Angulo P. Nonalcoholic fatty liver disease.N Engl J Med 2002; 346:1221-31. [2] Bruce R. Bacon .Genetic, Metabolic and Infiltrative diseases affecting the liver. Harrisons Internal Medicine 17th Edition; 303:1982. [3] Sui X ,Church TS ,Meriwether RA, et al. Uric acid and the development of metabolic syndrome in women and men. Metabolism 2008; 57:845-52. [4] Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia.Am J Med 2007; 120:442-7. [5] Nakagawa T, Tuttle KR, Short RA, et al.Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome.Nat Clin Pract Nephrol 2005; 1:80-6. [6] Carnethon MR,Fortmann SP,Palaniappan L, et al.Risk factors for progression to incident hyperinsulinemia: the Atherosclerosis Risk in Communities Study,1987-1988.Am J Epidemiol 2003;158:1058-67. [7] Masuo K, Kawaguchi H, Mikami H, et al.Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003; 42:474-80. [8]Dehghan A, van Hoek M, Sijbrands EJ, et al.High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008; 31:361-2. [9] Johnson RJ, Perez-Pozo SE, Sautin YY, et al.Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 2009; 30:96-116. [10] Lonardo A, Loria P, Leonardi F, et al. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease. A case-control study. Dig Liver Dis 2002; 34:204-11. [11] Li Y, Xu C, Yu C, et al. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol 2009; 50:1029-34. [12] Clark JM, Diehl AM. Defining non-alcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology 2003; 124:248-50. [13] Sanyal AJ. AGA technical review on non-alcoholic fatty liver disease. Gastroenterology 2002; 123:1705-25. [14] Scatarige JC, Scott WW, Donovan PJ et al: Fatty infilteration of the liver: ultrasonographic and computed tomographic co relation: J Ultrasound Med 1984; 3:9 [15] Chang Y, Ryu S, Sung E, et al. Higher concentrations of alanine aminotransferase within the reference interval predict non-alcoholic fatty liver disease. Clin Chem 2007; 53:686-92. [16] Mathiesen UL, Franzen LE, Aselius H, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis 2002; 34:516-22. [17] Shi JP, Fan JG, Wu R, Gao XQ, Zhang L, Wang H, et al.Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J Gastroenterol Hepatol 2008; 23:1419–25. [18] Li Y, Xu C, Yu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a crosssectional study. J Hepatol 2009; 50:1029–34. [19] Yong-Jae Lee, Hye-Ree Lee, Jung-Hyun Lee, Youn-Ho Shin and Jae-Yong Shim. Association between serum uric acid and non-alcoholic fatty liver disease in Korean adults. Clin Chem Lab Med 2010; 48(2):175–180. [20] Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986; 292:13–5.