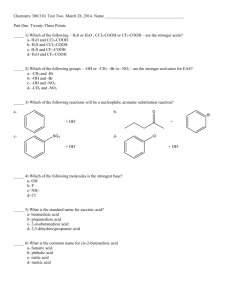
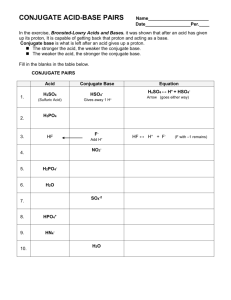
Acids and Bases
advertisement
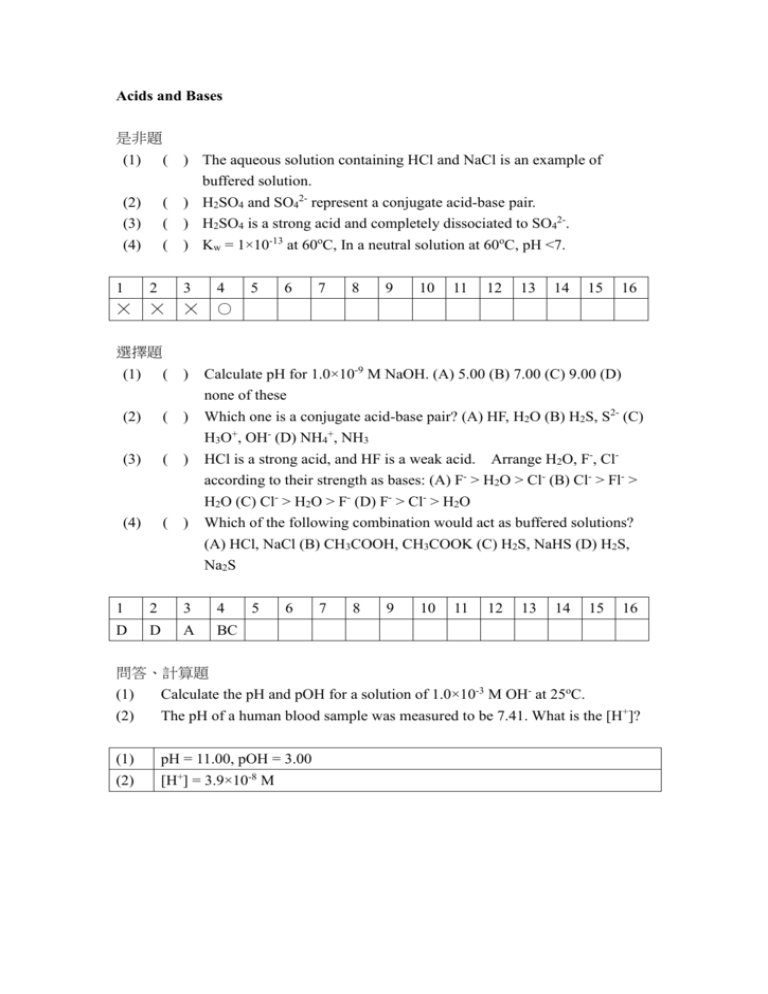
Acids and Bases 是非題 (1) ( ) The aqueous solution containing HCl and NaCl is an example of buffered solution. ( ) H2SO4 and SO42- represent a conjugate acid-base pair. ( ) H2SO4 is a strong acid and completely dissociated to SO42-. ( ) Kw = 1×10-13 at 60oC, In a neutral solution at 60oC, pH <7. (2) (3) (4) 1 2 3 4 ☓ ☓ ☓ ○ 5 6 7 8 9 10 11 12 13 14 15 16 選擇題 (1) ( ) (2) ( ) (3) ( ) (4) ( ) Calculate pH for 1.0×10-9 M NaOH. (A) 5.00 (B) 7.00 (C) 9.00 (D) none of these Which one is a conjugate acid-base pair? (A) HF, H2O (B) H2S, S2- (C) H3O+, OH- (D) NH4+, NH3 HCl is a strong acid, and HF is a weak acid. Arrange H2O, F-, Claccording to their strength as bases: (A) F- > H2O > Cl- (B) Cl- > Fl- > H2O (C) Cl- > H2O > F- (D) F- > Cl- > H2O Which of the following combination would act as buffered solutions? (A) HCl, NaCl (B) CH3COOH, CH3COOK (C) H2S, NaHS (D) H2S, Na2S 1 2 3 4 D D A BC 5 6 7 8 9 10 11 12 13 14 15 16 問答、計算題 (1) (2) Calculate the pH and pOH for a solution of 1.0×10-3 M OH- at 25oC. The pH of a human blood sample was measured to be 7.41. What is the [H+]? (1) pH = 11.00, pOH = 3.00 (2) [H+] = 3.9×10-8 M