Acid-base
advertisement

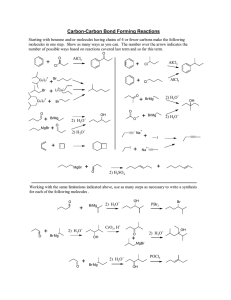
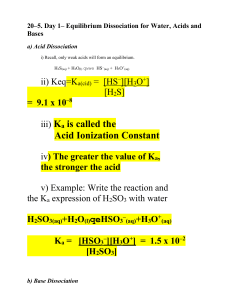
Strong and Weak Acids and Bases Information: When an acid is placed in water, hydronium ions are produced according to: HA (aq) + H2O H3O + (aq) + A- (aq) We write the equilibrium expression: Kc = [H3O+] [A-] [HA] [H2O] In almost all cases, the [H2O] does not change upon reaction with the acid (it is really large compared to the HA concentration) so it is incorporated into the value of Kc and given a special name (another special K!) as Ka. [H2O] = 1000g/L = 55 M 18g/mole Kc x 55 = Ka = [H3O+] [A-] [HA] You’ll find a table of Ka values at the back of your book. More information: A strong acid is one that is 100% dissociated in water. In other words, if 1.0 mole of acid is added to make a 1.0 L solution, the resulting [H3O+] is 1.0 M. List the strong acids here: (check your book!) Memorize this list. A weak acid is one that is significantly less than 100% dissociated in water. In weak acid solutions made from 1.0 mole of acid to make 1.0 L of solution, the [H3O+] is much less than 1.0 M. The relative strength of a weak acid can be determined by examining the value of Kz. The larger the Ka, the stronger the weak acid and the higher the [H3O+]. Questions: 1. A solution of nitrous acid contains: [HNO2] = 1.33 M [H3O+] = 0.026 M [NO2-] = 0.026 M a). Write a chemical equation that shows the reaction of HNO2 with water. Label the conjugate acid-base pairs. b). Calculate Ka c). Explain why [H3O+] = [NO2-] for this solution. d. Calculate the pH of this solution. 2. Determine the following for a solution of 1.0 M HCl a). [HCl] = b). [H3O+] = c). pH = Applying ICE tables to weak acids: 1).Write the reaction equation for when HOCl is added to water. 2). Write the Ka expression. 3). Ka = 2.9 x 10-8 Complete the table for 0.30 M HOCl HOCl H3O+ OCl- I C E Find the pH of this solution. Information When a base B is added to water, hydroxide ions are produced: B (aq) + H2O BH+ (aq) + OH- (aq) Based on what you know about Ka, write an expression for Kb for the above system. Problem: 1). Determine the [OH-] in 1.0 NH3 (aq) using the fact that Kb 1.8 x 10-5. a). Write the reaction equation for whn NH3 is added to water. b). Write the Kb expression. c). Construct and complete an ICE table. More information: Ka and Kb are related for conjugate acid base pairs. Complete the table: Acid HF Ka expression Conjugate Base HONO NH3 1. How are Ka , Kb , and Kw related? Kb expression Ka x Kb 2. Determine the numerical value of Ka and Kb in the table above. Look up the necessary values in your book. 3. Complete the table for conjugate acid-base pairs: Acid CH3COOH H2CO3 HONO NH4+ Ka 1.8 x 10-5 4.5 x 10-7 5.1 x 10-4 5.6 x 10-10 Base Kb












![Name: ______[KEY]______ Date: ______ Period: _____ WS Acids](http://s3.studylib.net/store/data/008944998_1-d9748ccda5f16e8a1324eb0f3ea7e450-300x300.png)