Appendix for Enzyme Endurance - Bio-Link
advertisement
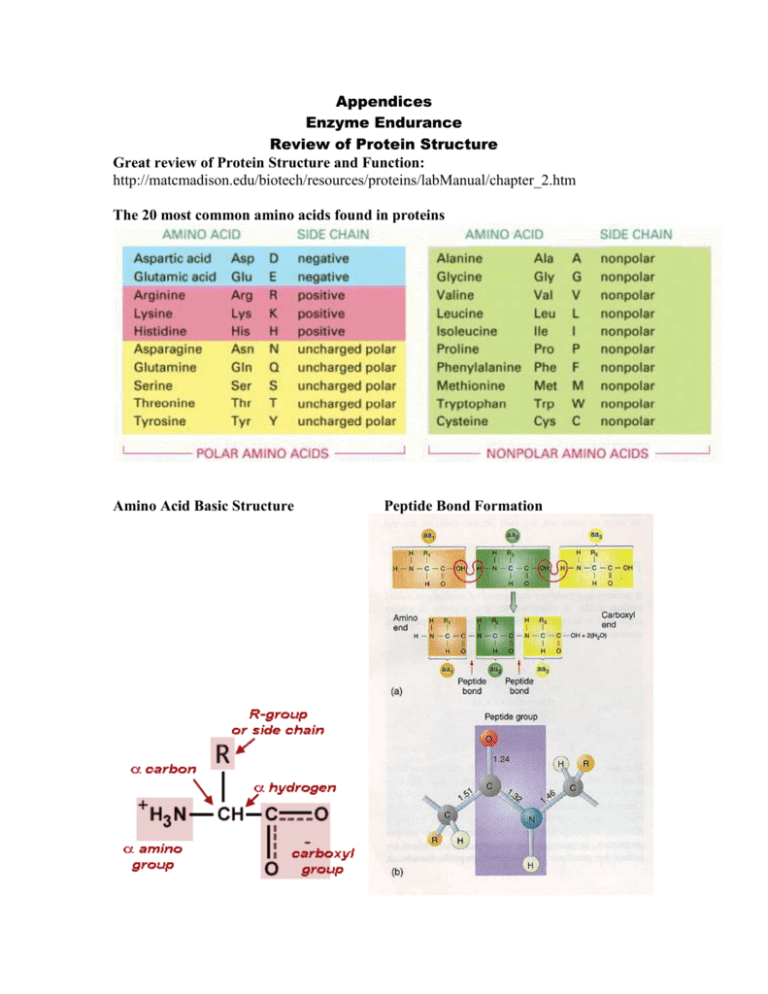
Appendices Enzyme Endurance Review of Protein Structure Great review of Protein Structure and Function: http://matcmadison.edu/biotech/resources/proteins/labManual/chapter_2.htm The 20 most common amino acids found in proteins Amino Acid Basic Structure Peptide Bond Formation Enzyme Endurance Notes on Enzymes Enzymes are usually proteins that first bind tightly to specific molecules, called substrates, and then catalyze the making and breaking of covalent bonds in these molecules. Background on Enzymes http://student.biology.arizona.edu At the active site of an enzyme, the amino acid side chains of the folded protein are precisely positioned so that they favor the formation of the high-energy transition states that the substrates must pass through. Active site of Lysozyme shown with substrate (green) bound. http://chemistry.umeche.maine.edu The three-dimensional structure of many proteins has evolved so that the binding of a small ligand can induce a significant change in protein shape. Most enzymes are allosteric proteins that can exist in two conformations that differ in catalytic activity, and the enzyme can be turned on or off by ligands that bind to a distinct regulatory site to stabilize either the active or the inactive conformation. The activities of most enzymes within the cell are strictly regulated. One of the most common forms of regulation is feedback inhibition, in which an enzyme early in a metabolic pathway is inhibited by it's binding to one of the pathway's end products. End product inhibition is negative feedback used to regulate the production of a given molecule. The initial substrate is a molecule that is altered in three steps by enzymes 1,2 and 3. The end product will combine with enzyme 1 to stop the reaction. www.okc.cc.ok.us Not all enzymes are protein in nature. RNA is also known to have catalytic activity, and catalytic RNA is termed a ribozyme. An atomic model of the full-length hammerhead ribozyme obtained by UCSC scientists using x-ray crystallography enabled them to describe how the structure catalyzes chemical reactions. Image: M. Martick and W. G. Scott, Cell (July 28, 2006) Enzyme Endurance Enzyme Types and Naming Conventions Enzyme Reaction Catalyzed Hydrolases General term for enzymes that catalyze a hydrolytic cleavage reaction. Nucleases Breakdown nucleic acids by hydrolyzing bonds between nucleotides. Proteases Breakdown proteins by hydrolyzing bonds between amino acids. Synthases General name used for enzymes that synthesize molecules in anabolic reactions by condensing two smaller molecules together. Isomerases Catalyze the rearrangement of bonds within a single molecule. Polymerases Catalyze the polymerization reactions such as the synthesis of DNA and RNA. Kinases Catalyze the addition of phosphate groups to molecules. Protein kinases are an important group of kinases that attach phosphate groups to proteins. Phosphatases Catalyze the hydrolytic removal of a phosphate group from a molecule. Oxido-Reductases General name for enzymes that catalyze reactions in which one molecule is oxidized while the other is reduced. Enzymes of this type are often called oxidases, reductases, and dehydrogenases. ATPases Hydrolyze ATP. Many proteins with a wide range of roles have an energy-harnessing ATPase activity as part of their function, for example, motor proteins such as myosin and membrane transport proteins such as the sodium-potassium pump. Enzyme names typically end in "ase", with the exception of some enzymes, such as pepsin, trypsin, thrombin, lysosyme, which were discovered before the convention became generally accepted at the end of the nineteenth century. The common name of an enzyme usually indicates the substrate and the nature of the reaction catalyzed. For example, citrate synthase catalyzes the synthesis of citrate by the addition of acetyl CoA to oxaloacetate.










