APC iSL4
advertisement

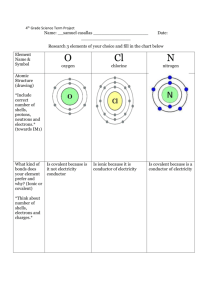
iSL#4 Name: ________________________ Covalent Bonding in Chlorine and Nitrogen Open the Odyssey Molecular Labs “ML 13: Covalent Bonding in Chlorine and Nitrogen” Objectives To examine the formation of a chlorine molecule from its constituent atoms. To determine the equilibrium bond length and bond strength from a potential energy diagram. To juxtapose the actual electron density of the bonded molecule with symbolic representations of covalent bonds. To compare the bond in molecular nitrogen with that in molecular chlorine. Procedure and Analysis Molecular chlorine, Cl2, is a typical example of a covalently bonded molecule. A sequence of calculated electron densities is available that shows two chlorine atoms as they approach each other: Erelative = 0 kJ/mol The shown energy value is taken relative to the energy of the dissociated atoms. It varies between negative values for favorable ("bonded") distances and positive values for unfavorable distances. 1. Generate a plot of the energy as a function of distance: From the View menu, select Plots. From the Plots menu (upper left corner), select XY Plot.... For the "X Axis," select Cl-Cl Distance (pm). For the "Y Axis," select Relative Energy (kJ/mol). Click Next. Request a Scatter Plot and click on Finish. (You can make the entire plot bigger by dragging the right-side boundary of the plot area.) What do you find for the equilibrium bond length of chlorine? 2. Apart from the fact that it corresponds to a minimum of the energy, is there anything really unique about the equilibrium bond distance? Provided that energy is available, is the molecule "allowed" to be at distances other the equilibrium bond length? 3. The dissociation energy is the difference in energy between the bonded molecule and the completely separated atoms. What is the calculated dissociation energy of chlorine? Page 1 of 2 © 2004-2009 Wavefunction, Inc. 4. Nitrogen is also known to form a covalently bonded diatomic molecule, N 2, albeit with a rather short bond. Here are some snapshots of the electron density for different distances of the two atoms: 540 pm 150 pm 105 pm 400 pm 135 pm 90 pm 175 pm 110 pm 83 pm Generate a second plot for the distance-energy relationship of this molecule: From the Plots menu, select XY Plot.... For the "X Axis," select N-N Distance (pm). For the "Y Axis," select Relative Energy (kJ/mol). Click Next. Request a Scatter Plot and click on Finish. What do you find for the equilibrium bond length of nitrogen? 5. What is the dissociation energy of nitrogen? 6. Nitrogen has a "triple" bond, while chlorine has a "single" bond. If we ignore the fact that we are comparing molecules whose atoms are different, what is the ratio of the two bond strengths? 7. Do the curves for the energy as a function of distance look fundamentally different for chlorine and nitrogen? Page 2 of 2 © 2004-2009 Wavefunction, Inc.