An introduction to acids and bases
advertisement

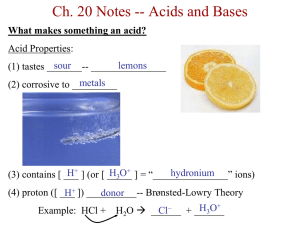
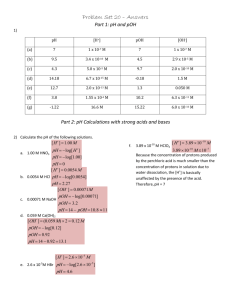
An introduction to acids and bases The grade 10 definitions: Acid: sour, conductor, red in litmus Base: bitter, conductor, slippery, blue in litmus Arrhenius’ definitions: Acid: produce H+ ions in aqueous solutions Bases: produce OH- ions in aqueous solutions **these two definitions can explain most acids and bases but not those like NH3 ** Bronsted-Lowry theory: Acids: donate protons Bases: accept protons Examples : 1. 2. ALSO, some substances can donate OR accept a proton. These are called amphoteric. (they can act like acids OR bases) Example: Reversible acid-base reactions: Example: - 2 acids: -2 bases: -substances whose formulas differ by a single proton are called CONJUGATE acid-base pairs: A general term for this: Which simplifies to: The autoionization of water…takes place in all water samples Looks like: -this is why water has a slight conductivity We can simplify to: And make an equilibrium equation: And then simplify again because the concentration of water molecules is always 55.6mol/L: Measurements show that the concentrations of H+ and OH- are both 1.0 x 10-7 at SATP so: We use calculate Kw to calculate the H+ and OH- concentrations in aqueous solution of a strong or a weak base at SATP if the concentration of the other is known: Strong acids -ionize quantitatively (completely) in water to make H+ -assume 100% ionization -include: HCl, HBr, H2SO4 , HNO3 and H3PO4 -one hydrogen is called monoprotic, two is diprotic, three is triprotic Example: Calculate the concentration of hydroxide ions in a 0.15 mol/L solution of HCl. Strong bases -ionize completely in water to make OH-assume 100% ionization -examples include group 1 and group 2 hydroxides ( Example: calculate the concentration of hydroxide in a 0.25 mol/L solution of barium hydroxide, a strong base. Hydrogen ion concentration and pH pH = -log[H+(aq) ] Therefore, we can calculate pH if we have [H+(aq) ] Example: calculate the pH of a solution with a concentration of H+ ions of 4.7 x 10-11 mol/L ) In pure water: Therefore: To calculate concentration of hydrogen ions from pH use this formula: Example: convert pH 10.33 to concentration of hydrogen ions pOH and pKw pOH is a way to describe hydroxide ion concentrations pOH = -log[OH-(aq) ] and [OH-(aq) ] = 10-pOH Example: Calculate pOH of a solution with a hydroxide ion concentration of 3.0 x 10-6 mol/L The relationship between pH and pOH: pH + pOH = pKw So… pH + pOH = Example: What is the pOH of a solution whose pH is measured to be 6.4? …just a reminder that you can measure pH using indicators (natural or synthetic) or pH meters (some actually give pretty accurate readings… others are annoying) The pH of strong acids Example: Calculate the pH, pOH and [OH-(aq) ] of a 0.04 mol/L solution of HNO3 1. write dissociation equation 2. calculate pH 3. calculate pOH 4. use pOH to calculate [OH-(aq) ] The pH of strong bases: Example: calculate the pH of a 0.02mol/L NaOH solution *note: you could also be asked to calculate hydrogen ion concentrations, you could also be asked to start with grams and convert to moles, find concentration and then go from there.*