Supplementary Material - Proceedings of the Royal Society B
advertisement

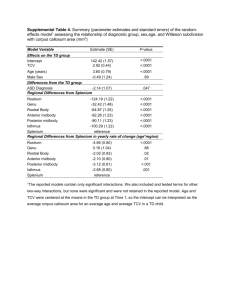
SUPPLEMENTARY METHODS Sample Collection We captured 212 individuals from five subspecies -- Alabama, Perdido Key, Santa Rosa Island, Choctawhatchee and Saint Andrew beach mice -- using Sherman live traps (Table 1; S1). At each sampling site, traps were set every 10 m and a minimum of five trap lines were set in the frontal dunes, where mice are most abundant. For each individual captured, we sampled a 2-mm tail tip and quantified color pattern (see below), except in 16 samples for which we had only genetic data. We also collected multiple sand samples from within the range of each of the subspecies. Sand samples were collected at the beginning and end of each trap line, and were representative of the range of sand color at each site. For each sand sample, we measured brightness following the methods of Mullen and Hoekstra (2008). Briefly, we used a USB2000 (Ocean Optics, Dunedin, FL) spectrophotometer to capture reflectance across the light spectrum visible to most predators (300-700 nm; Bennett and Cuthill 1994). We recorded measurements in triplicate using the program OOIBASE 32 (Ocean Optics) and calculated brightness following Endler (1990). For each sampling site, we averaged the brightness of the sand samples. Measuring color pattern To measure color pattern, we scored 12 traits across the body, which showed the most difference in pigmentation among individuals (Fig. S1). For nine traits, we used a three-category system based on the pigmentation of individual hairs within each body region: no visible pigment, partially pigmented or fully pigmented. For three additional traits, we used a fivecategory system to score the distribution of pigmented hairs across the flank, rump, and tail (as opposed to the extent of pigmentation on individual hairs). Methods follow those outlined in Steiner et al. (2007). Genetic variation We collected genetic data from three different types of markers: microsatellite loci, mitochondrial DNA (mtDNA) sequences and single nucleotide polymorphisms (SNPs) in nuclear genes. Microsatellite markers: We genotyped 212 individuals using 20 microsatellite markers (Table S2). Primer sequences and reaction conditions are reported in Mullen et al. (2006). All 1 microsatellite loci were run on an ABI 3100 sequencer, and genotypes were scored using GENEMAPPER v.3.5 (Applied Biosystems). MtDNA sequencing: For 70 individuals (10-12 individuals from each subspecies and two outgroups), we sequenced two mitochondrial regions (ND3-COIII and the control region). We co-amplified ND3 and COIII using previously published primer sequences (ND3-H and CO3-L; Hoekstra et al. 2004) and a 58.8°C annealing temperature. Separately, we amplified the control region (PL276-R and PL792-F; Van Zant and Wooten 2007) with an annealing temperature of 54.6°C. Cycle sequencing was performed using the amplification primers. We then concatenated the resulting sequences and aligned them using SEQUENCHER v.4.1 (Gene Codes Corp). Nuclear loci: We genotyped single nucleotide polymorphisms (SNPs) from eight nuclear genes with no known effect on pigmentation in the same 70 individuals from which we sequenced mtDNA. For five of the loci, CAMK4, MPO, OAT, PAH and RB1, primer sequences and PCR reaction conditions are reported in Lyons et al. (1997); primer sequences and conditions for the remaining three loci, phosducin-like protein, riboprotein-1 and cd81, are reported in Steiner et al. (2007). Following amplification, we identified a polymorphism in each locus and designed a Taqman assay for SNP genotyping. Finally, we genotyped all 212 samples for a single SNP located in the melanocortin-1 receptor (Mc1r) that was previously implicated in color variation in P. polionotus (Hoekstra et al. 2006). All Taqman assays were conducted in standard conditions, visualized on an ABI 7000 and analyzed using the Allelic Discrimination Assay procedure in SDS v.1.1. REFERENCES Bennett, A. T. D. and I. C. Cuthill. 1994. Ultraviolet vision in birds: What is its function? Vision Research 34:14711478. Endler, J. A. 1990. On the measurement and classification of color in studies of animal color patterns. Biological Journal of the Linnean Society 41:315-352. Hoekstra, H. E., K. E. Drumm and M. W. Nachman. 2004. Ecological genetics of adaptive color polymorphism in pocket mice: geographic variation in selected and neutral genes. Evolution 58:1329-1341. Hoekstra, H. E., R. J. Hirschmann, R. A. Bundey, P. A. Insel and J. P. Crossland. 2006. A single amino acid mutation contributes to adaptive beach mouse color pattern. Science 313:101-104. Lyons, L. A., T. F. Laughlin, N. G. Copeland, N. A. Jenkins, J. E. Womack and S. J. O'Brien. 1997. Comparative anchor tagged sequences (CATS) for integrative mapping of mammalian genomes. Nature Genetics 15:47-56. Mullen, L. M., R. J. Hirschmann, K. L. Prince, T. L. Glenn, M. J. Dewey and H. E. Hoekstra. 2006. Sixty polymorphic microsatellite markers for the oldfield mouse developed in Peromyscus polionotus and P. maniculatus. Molecular Ecology Notes 6:36-40. Mullen, L. M. and H. E. Hoekstra. 2008. Natural selection along an environmental gradient: a classic cline in mouse pigmentation. Evolution 7:1555-1569. Steiner, C. C., J. N. Weber and H. E. Hoekstra. 2007. Adaptive variation in beach mice produced by two interacting pigmentation genes. PLoS Biology 5:1880-1889. Van Zant, J. L. and M. C. Wooten. 2007. Old mice, young islands and competing biogeography hypotheses. Molecular Ecology 16:5070-5083. 2 Table S1. Subspecies, sample size, collection site (latitude/longitude) and identification numbers for each individual used in this study. Alabama beach mice (P. p. ammobates), N = 46. Gazebo Unit (30.229978, -87.814703): ABM B32, 425, 426, 442, 473, 501, 503, 505, 615, 631, 683, 1191, 2018, 2107, 2298, 2333, 2618, 2624, 2766, 3001, 4637. Perdue Unit (30.229259, -87.831163): ABM 182, 254, 438, 481, 487, 547, 573, 594, 602, 617, 626, 660, 719, 1200, 1201, 1240. Dunes Development Unit (30.227292, -87.995922) ABM 3, 11. Fort Morgan Unit (30.225000, - 88.018889) ABM 142, 573, 3077, 7019, 8021, 8216. Transition Grid (30.231911, 87.819703): ABM 720. Perdido Key beach mice (P. p. trisyllepsis), N = 18. Perdido Key State Park (30.293710, -87.463557): PKBM 05-01 to 05-08. Johnson Beach (30.325827, -87.319856): PKBM 241, 278, 283, 311, 315, 329, 330, 332, 338, 340. Santa Rosa Island beach mice (P. p. leucocephalus), N = 42. Eglin Air Force Base- Santa Rosa Island (30.398649, -87.694538): SRIBM 05-01 to 05-09. Gulf Islands National Seashore Fort Pickens (30.328795, -87.299345): SRIBM 06-10 to 0642. Choctawhatchee beach mouse (P. p. allophrys), N = 65. Shell Island (30.077795, -85.647814): CBM 06-40, 06-46. Tyndall Air Force Base (30.062053, -85.614280): CBM 05-1 to 05-20 and 06-41 to 06-45. Topsail Hill State Park (30.359951, -86.282125): CBM 05-21 to 05- 31, 05-50, 05-51, 313, 404, 405, 409, 424-426, 428, 430-434, 438, 443, 445, 446, 452-455, 457, 460, 470, 471, 497. Eastern end of West Crooked Island (30.014103, -85.553717): CBM 05-32. Saint Andrew beach mouse (P. p. peninsularis), N = 39. East Crooked Island (29.957198, -85.462412): SABM 05-1 to 05-35. St. Joseph Peninsula State Park (29.872629, -85.394240): SABM 06-36 to 06-39. 3 Table S2. Genetic diversity for microsatellite loci in five beach mouse subspecies. Number of alleles (Na), observed heterozygosity (HO) and expected heterozygosity (HE) are shown. Asterisk (*) indicates loci showing a significant departure from HWE. Site ABM PKBM SRIBM CBM SABM Bw2- Bw2- Bw2- Bw3- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Bw4- Po- Po- 25 76 110 12* 1 5 8 45* 63* 74* 93 110 112* 137 143 186 200 SREL 17* 31* Na 9 5 5 3 11 5 3 9 2 9 6 6 8 8 3 5 10 5 11 12 HO 0.46 0.44 0.64 0.36 0.94 0.34 0.23 0.61 0.07 0.23 0.46 0.40 0.71 0.26 0.59 0.29 0.64 0.50 0.26 0.58 HE 0.70 0.40 0.71 0.49 0.81 0.36 0.51 0.82 0.41 0.46 0.69 0.54 0.80 0.76 0.49 0.53 0.75 0.57 0.66 0.85 Na 5 8 2 3 4 4 1 6 3 5 3 8 5 5 2 3 4 2 9 5 HO 0.44 0.61 0.12 0 0.72 0.44 0 0.50 0.78 0.06 0.50 0.24 0.35 0.28 0.06 0.39 0.20 0.94 0.06 0.72 HE 0.57 0.79 0.11 0.24 0.58 0.45 0 0.72 0.65 0.42 0.48 0.82 0.40 0.76 0.05 0.33 0.39 0.50 0.85 0.69 Na 2 7 3 4 5 3 3 6 4 7 8 8 8 8 4 7 11 6 6 17 HO 0.27 0.19 0.23 0.20 0.73 0.40 0.38 0.32 0.02 0.17 0.91 0.71 0.45 0.85 0.20 0.41 0.71 0.46 0.11 0.71 HE 0.23 0.71 0.24 0.34 0.75 0.34 0.50 0.56 0.49 0.34 0.81 0.66 0.82 0.68 0.18 0.47 0.85 0.80 0.60 0.84 Na 7 5 6 4 11 2 3 11 2 3 9 10 12 6 3 3 9 4 4 10 HO 0.62 0.46 0.31 0.02 0.47 0.23 0.18 0.50 0 0.02 0.63 0.63 0.42 0.55 0.03 0.06 0.55 0.28 0.06 0.65 HE 0.71 0.45 0.68 0.12 0.52 0.20 0.43 0.75 0.41 0.09 0.75 0.58 0.75 0.69 0.03 0.09 0.63 0.63 0.11 0.80 Na 8 6 2 5 7 4 3 9 4 4 6 7 7 8 5 4 15 6 4 3 HO 0.40 0.41 0 0 0.58 0.31 0.45 0.25 0.03 0.26 0.26 0.26 0.38 0.30 0.18 0.90 0.58 0.61 0.05 0.08 HE 0.71 0.48 0.05 0.64 0.64 0.62 0.45 0.70 0.42 0.28 0.40 0.54 0.63 0.35 0.39 0.57 0.82 0.64 0.34 0.28 4 Table S3. Statistical differences among subspecies in 12 pigmentation traits, calculated using MANOVA. R2 and p-values are shown. r2 P-value Rostrum 0.696 < 0.0001 Eye-whisker 0.648 < 0.0001 Cheek 0.873 < 0.0001 Eye-ear 0.556 < 0.0001 Btwn eyes 0.435 < 0.0001 Eyebrows 0.616 < 0.0001 Earbase 0.740 < 0.0001 Ventrum 0.771 < 0.0001 Ankle 0.745 < 0.0001 Shadow 0.825 < 0.0001 Rump 0.791 < 0.0001 Tail 0.630 < 0.0001 5 SUPPLEMENTARY FIGURE LEGENDS Figure S1. Cartoon of a beach mouse highlighting the 12 pigmentation traits measured. 6



![PERSONAL COMPUTERS CMPE 3 [Class # 20524]](http://s2.studylib.net/store/data/005319327_1-bc28b45eaf5c481cf19c91f412881c12-300x300.png)