Answers
advertisement

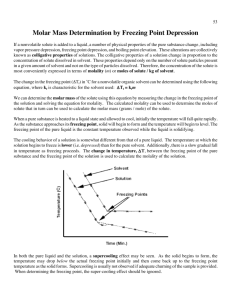
Unit 6 - Solutions Section 3 – Colligative Properties and Non-Ideal Behavior 1. How does increasing the concentration of a nonvolatile solute in water affect the following properties of the water? a. Vapor pressure Increasing the concentration of a nonvolatile solute will DECREASE the vapor pressure of the solution. b. Freezing point Increasing the concentration of a nonvolatile solute will DECREASE the freezing point of the solution. c. Boiling point Increasing the concentration of a nonvolatile solute will INCREASE the boiling point of the solution. d. Osmotic pressure Increasing the concentration of a nonvolatile solute will INCREASE the osmotic pressure of the solution. 2. If you have a solution of 10 grams of glucose (C6H12O6) added to 1 L of water, and 10 grams of sucrose (C12H22O11) added to 1 L of water. Are the vapor pressures over the two solutions the same? Vapor pressure is dependent upon concentration of the solution. The glucose solution is more concentrated because it has a lower mass than the sucrose so there are more particles in 10 grams of glucose than 10 grams of sucrose. 3. How many grams of ethylene glycol (C2H6O) must be added to 1.00 kg of water to produce a solution that freezes at -5.00 ºC? T f K f m 5 1.86m 2.688 mol 2.688 mol 46 g 124 g 1 1 mol 4. The molar mass of an unknown solid, which is nonvolatile and a nonelectrolyte, is to be determined by the freezing-point depression method. The pure solvent used in the experiment freezes at 10 °C and has a known molal freezing-point depression constant of, Kf. Assume that the following materials are also available: test tubes, beaker, stirrer, stopwatch, pipette, graph paper, thermometer, hot-water bath, balance, ice. a. Sketch the cooling curves for the pure solvent vs. time and the solution vs. time as each is cooled from 20 °C to 0.0 °C. b. Information from these graphs may be used to determine the molar mass of the unknown solid. i. Describe the measurements that must be made to determine the molar mass of the unknown solid by this method. Measure the mass of the solute, the solvent, and the solution. Measure the change in freezing temperature of the solution. ii. Show the setup for the calculation that must be performed to determine the molar mass of the unknown solid from the experimental data. Using the freezing point depression formula: ∆T = Kf m, solve for molality (m). Then using m = mol solute/kg solvent, solve for mol solute. Then using mol = g/molar mass, solve for molar mass of solute. iii. Explain how the differences between the two graphs in part a can be used to obtain information needed to calculate the molar mass of an unknown solid. The differences between the two graphs in part a are used to determine the ∆T for the solution. c. Suppose that during the experiment a significant but unknown amount of solvent evaporates from the test tube. What effect would this have on the calculated value of the molar mass of the solid (i.e. too large, too small, or no effect)? Justify your answer. This would affect the calculated value for the molar mass of the solid. If there is less solvent, the freezing point would seem lower than it actually would be. If the freezing point seems lower, then the molality would seem higher. An increase in molality would cause moles of solute to seem higher. This would make the molar mass seem lower. d. Show the setup for the calculation of the percentage error in a student’s result if the student obtains a value of 126 g/mol for the molar mass of the solid when the actual value is 120. g/mol. Percent Error accepted - experiment al 120 126 100% 100% accepted 120