RANGER COLLEGE COURSE NUMBER AND TITLE: Chemistry
advertisement

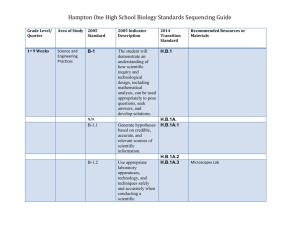
RANGER COLLEGE COURSE NUMBER AND TITLE: Chemistry 1411 General Chemistry I CREDIT HOURS: 3 HRS/WK LECTURE & 3 HRS/WK LAB LEC/LAB/HRS/WK COMBINATION: 4 credit hours total Name of Instructor: Kimberlea M. Adams Office: Stephenville High School, Room 513 Office Hours: 3:00 – 3:45 p.m. Monday- Friday Office Phone: 254-968-4141 E-Mail: kadams@rangercollege.edu & kimberlea.adams@sville.us I. CATALOG DESCRIPTION General principles, problems, fundamental laws, and theories. Course content provides a foundation for work in advanced chemistry and related sciences. Recommended for preprofessional and science majors. II. COURSE GOAL Students completing General Chemistry will understand the basics of atomic theory, and the nature of matter relative to quantifying chemical reactions. Students will learn the ways in which matter reacts to form chemical compounds and the rates and dynamics of these reactions. Students will understand the basic methods of performing qualitative analysis. III. COURSE CONTENT 1. An introduction to the basic classifications of matter and the types of changes matter undergo, the scientific method, units of measurements and the treatment of significant figures in measured values and dimensional analysis. (Ch. 1) Labs: a) Basic Laboratory Techniques, Laboratory Safety and Computer Aided Graphing b) Determination of the Mass, Volume and Density of Unknown Solids and Liquids 2. An introduction to the periodic table, chemical symbols, chemical formulas, and nomenclature, including a descriptive study on periodic trends. (Ch. 2, 3 & 7) Labs: a) It’s in the Cards: Build a Periodic Table Based on Properties (Dry Lab) 3. The representation of various chemical reactions types by chemical equations and the stoichiometric analysis of quantitative information derived from chemical formulas and equations such as moles, empirical formulas, percent composition, and percent yield. (Ch. 3 & 4) Labs: a) Mass of Excess Reactant b) Determination of an Empirical Formula 4. An in depth look at the composition of the basic building blocks of matter, atoms, and the theories, past and current, concerning atomic and molecular structure. (Ch. 2, 6 & 7) Labs: a) VSEPR Theory: Molecular Model Building of Organic Compounds Using Ball And Stick Model and Gum Drops (Dry Lab) b) Emission Spectra and Atomic Structure 5. A thorough description of the different types of chemical bonds which hold atoms together in compounds. (Ch. 8 & 9) Labs: a) Comparison of Physical Properties of Common Ionic and Molecular Compounds b) Identification of Two Substances from Physical Properties 6. A description of the different types of forces which hold compounds together in materials and the quantitative relationships between energy changes and chemical reactions. (Ch. 5) Labs: a) Constant Pressure Calorimetry b) Determination of an Enthalpy Change Associated with a Chemical Reaction 7. A comprehensive study of the three basic phases of matter: solids, liquids and gases. (Ch. 10 & 11) Labs: a) Behavior of Gases 8. The explanation of the physical laws and kinetics which govern the properties of gases, and the application of these laws such as the combined gas law, ideal gas law, Avogadro’s Principle, Dalton’s and Graham’s Laws . (Ch. 10) Labs: a) Determination of the Molar Volume of a Gas b) Analysis of a Potassium Chlorate/Potassium Chloride Mixture Using the Ideal Gas Law IV. REQUIRED BACKGROUND/PREREQUISITES Course Prerequisites: two units of high school algebra and a passing score on the math section of THEA test or equivalent alternative test. TEXTBOOK (S); READINGS; MATERIALS Text: Brown, Theodore E., H. Eugene LeMay, and Bruce E. Bursten, et al. Chemistry: The Central Science. 12th Edition (2012). Upper Saddle River, NJ: Prentice Hall. (required for homework and class) Solutions Manual: Brown, Theodore E., H. Eugene LeMay, and Bruce E. Bursten, et al. Solutions, Chemistry: The Central Science. 12th Edition (2012). Upper Saddle River, NJ: Prentice Hall. (Optional) Calculator: Scientific or graphing calculator (required for homework and class) V. METHODS OF INSTRUCTION Material will be presented in a variety of teaching styles. Chapter notes will be given via power point and smart board lecture presentations. Time will be given for classroom discussions and questions and answers. Some small group work will be done in class to build collaborative study groups. Some of these laboratory exercises will require multiple days in a row and thus the lecture schedule will be adjusted accordingly. Some of the laboratory exercises will be short enough to accompany a lecture. Be prepared to stay the entire class time both nights. VI. EXEMPLARY EDUCATIONAL OBJECTIVES – NATURAL SCIENCES (N) The objective of the study of a natural sciences component of a core curriculum is to enable the student to understand, construct, and evaluate relationships in the natural sciences, and to enable the student to understand the bases for building and testing theories. N-1. To understand and apply method and appropriate technology to the study of natural sciences. N-2. To recognize scientific and quantitative methods and the differences between these approaches and other methods of inquiry and to communicate findings, analyses, and interpretation both orally and in writing. N-3. To identify and recognize the differences among competing scientific theories. N-4. To demonstrate knowledge of the major issues and problems facing modern science, including issues that touch upon ethics, values, and public policies. N-5. To demonstrate knowledge of the interdependence of science and technology and their influence on, and contribution to, modern culture. VIII. BASIC INTELLECTUAL COMPETENCIES B-1 READING: Reading at the college level means the ability to analyze and interpret a variety of printed materials. B-2 WRITING: Competency in writing is the ability to produce clear, correct, and coherent prose adapted to purpose, occasion, and audience. B-3 SPEAKING: Competence in speaking is the ability to communicate orally in clear, coherent, and persuasive language appropriate to purpose, occasion, and audience. B-4 LISTENING: Listening at the college level means the ability to analyze and interpret various forms of spoken communication. B-5 CRITICAL THINKING: Critical thinking embraces methods for applying both qualitative and quantitative skills analytically and creatively to subject matter in order to evaluate arguments and to construct alternative strategies. B-6 COMPUTER LITERACY: Computer literacy at the college level means the ability to use computer-based technology in communicating, solving problems, and acquiring information. IX. COURSE OBJECTIVES Knowledge Objectives: Upon completion of this course: 1. Students will be able to demonstrate a satisfactory understanding of chemical symbols, chemical nomenclature, chemical reactions as represented by chemical equations, and stoichiometry. (N-1, N-2, N-3) (B-1, B-2, B-4, B-5) 2. Students will be able to demonstrate a satisfactory understanding of aqueous solutions, electrolytes, solubility, and important types of chemical reactions in solution. (N-1, N-2, N-3) (B-1, B-2, B-4, B-5) 3. Students will be able to demonstrate a satisfactory understanding of the relationship between energy and chemical reactions. (N-1, N-2, N-3) (B-1, B-2, B-4, B-5) 4. Students will be able to demonstrate a satisfactory understanding of the structure of matter, the electronic structure of the atom, and the relationship of electronic structure to chemical properties and the Periodic Table. (N-1, N-2, N-3) (B-1, B-2, B-4, B-5) 5. Students will be able to demonstrate a satisfactory understanding of different types of chemical bonds which bind atoms together. (N-1, N-2, N-3) (B-1, B-2, B-4, B-5) 6. Students will be able to demonstrate a satisfactory understanding of the physical laws which govern the properties of gases and atmospheric chemistry. (N-1, N-2, N-3) (B-1, B-2, B-4, B-5) 7. Students will be able to demonstrate a satisfactory understanding of the solid and liquid states of matter. (N-1, N-2, N-3, N-5) (B-1, B-2, B-4, B-5) Skill Objectives: Upon completion of this course: 1. Students will be knowledgeable about chemical laboratory safety. (N-1, N-2, N-3, N-5) (B1, B-2, B-4, B-5) 2. Students will be able to perform basic chemical observations and measurements. (N-1, N-2, N-3, N-5) (B-1, B-2, B-4, B-5, B-6) 3. Students will utilize dimensional analysis with correct significant figures. (N-1, N-2, N-3, N-5) (B-1, B-2, B-4, B-5) Value Objectives: Upon completion of this course: Students will have an appreciation of the Scientific Method and the role of Chemistry in modern society. (N-1, N-2, N-3, N-4, N-5) (B-1, B-2, B-4, B-5, B-6) X. COURSE CALENDAR TENTATIVE COURSE SCHEDULE Day/Date Lecture Topic T/January 14 Chapter 1 Introduction: Matter and Measurement R/January 16 Lab: Determination of Density T/January 21 Chapter 2 Atoms, Molecules, and Ions R/January 23 Homework Ch. 1 & 2 T/January 28 Complete Ch. 2 R/January 30 Test Ch. 1 & 2 T/February 4 Chapter 3 Stoichiometry: Calculations R/February 6 with Chemical Formulas and Equations T/February 11 Lab: Mass-Mass R/February 13 Con’t Ch. 3 T/February 18 Test Ch. 3 R/February 20 Chapter 4 Aqueous Reactions and T/February 25 Solution Stoichiometry R/February 27 Lab: Aluminum Wire as an Excess Reactant T/March 4 Test Ch. 4 R/March 6 Chapter 5 Thermochemistry T/March 18 Con’t. Ch. 5 R/March 20 Lab: Endo. v. Exo. Reactions T/March 25 Test Ch. 5 R/March 27 Chapter 6 Electronic Structure of Atoms T/April 1 Lab: Flame Test Complete Ch. 6 R/April 3 Chapter 7 Periodic Properties of the Elements T/April 8 Lab: It’s in the Card’s R/April 10 Complete Ch. 6 & 7 T/April 15 Test Ch. 6 & 7 R/April 17 Chapter 10 Gases T/April 22 Complete Ch. 10 R/April 24 Lab: Properties of Gases T/April 29 Gas Laws Quiz R/May 1 Semester Exam Review/Make-up Lab T/May 6 Comprehensive Final Exam XI. COURSE/CLASSROOM POLICIES The student learner is expected to attend and participate in all class and laboratory activities. Respectful and mature behavior is required by all students. The class meets twice weekly throughout the semester and includes lab and lectures. Hands-on lab experiences are embedded throughout the semester to align with course content. Some labs will take the entire class time while others will be paired with lecture. Dates are subject to change. Attendance of all classes and labs at 90% is required for credit to be given for this course. Make-up labs will be scheduled through the instructor and completed before or after class at a time agreed upon by the instructor and student. Only one lab make-up will be provided per student. Students will follow the laboratory safety procedures handed out to them on the first day of lab. Any misbehavior or intentional violation of safety rules will result in the student being asked to leave the laboratory area. Most of the chapters in the text are completed in a sequential order. A primary goal of this class is to encourage students to develop a sense of self and group accountability. The students are required to purchase texts, solution manuals are optional, and labs will be provided. All students are asked to maintain a portfolio/notebook in which to keep their completed work including quizzes and laboratory reports. When each new topic is introduced the correlated homework problems are assigned from the textbook. These assignments are meant to be practice for the quizzes and tests so answers are provided for feedback purposes. Many students will try, in the beginning, just to “look” over the homework, but quickly learn to work within a study group to solve the problems. Students are often asked to share and defend their solutions with the class. Students are encouraged to make contributions to problem-solving in this way to reinforce that the teacher is not the only one who can work the problem. Remember, I am available most days for tutorial help. Check in at the front vestibule of the high school with your driver’s license. You will be issued a picture ID. If you call or email me ahead of time I can meet you. Students are required to submit a detailed, comprehensive report of each laboratory experiment. These reports include hypothesis, procedure, collected data and observations, calculations and a valid conclusion. Some experiments are done individually, but most are done with a partner. Partners are rotated often to keep ideas and experiences varied and fresh. Both members of the group are required to manipulate the laboratory equipment and record their own observations throughout the course of the experiment. Ranger College provides a variety of services for students with learning and/or physical disabilities. The student is responsible for making the initial contact with the Ranger College Counselor. It is advisable to make this contact before or immediately after the semester begins. XII. ASSESSMENT (Grading Procedure) Grades for the course are calculated using the following guidelines: 25 percent--daily work (quizzes, homework problems from the text, laboratory write-ups), 50 percent-- lecture exams (multiple choice questions, short answer and problems), 25 percent-- comprehensive final exam. No make-up tests will be given. The lowest test grade will be dropped. Any academic dishonesty on labs, quizzes or exams will result in a zero on that assignment. Letter grades will be assigned as follows: 90 – 100 earns an A, 80 – 89 B, 70 – 79 C, XIII. 60 – 69 D, below 60 F NON-DISCRIMINATION STATEMENT Admission, employment, and program policies of Ranger College are non-discriminatory in regard to race, creed, color, sex, age, disability, and national origin. XIV. RECEIPT OF SYLLABUS FORM Please be sure to sign and return the attached form to me by the fifth class day. RECEIPT OF SYLLABUS “I have received and understand the information in the syllabus for General Chemistry, 1411 and I agree to abide by the stated policies.” Legibly print the following information: Name:__________________________ Date:___________________________ Signature of Student:____________________________________ In a short paragraph or two please answer the following: Why did you take dual credit General Chemistry? What are your career goals? Where do you plan to attend college? What is your favorite course (other than Chemistry, of course)? Why is it your favorite?