Supplemental Data (SD) SD1. FGF-2 pre
Supplemental Data (SD)
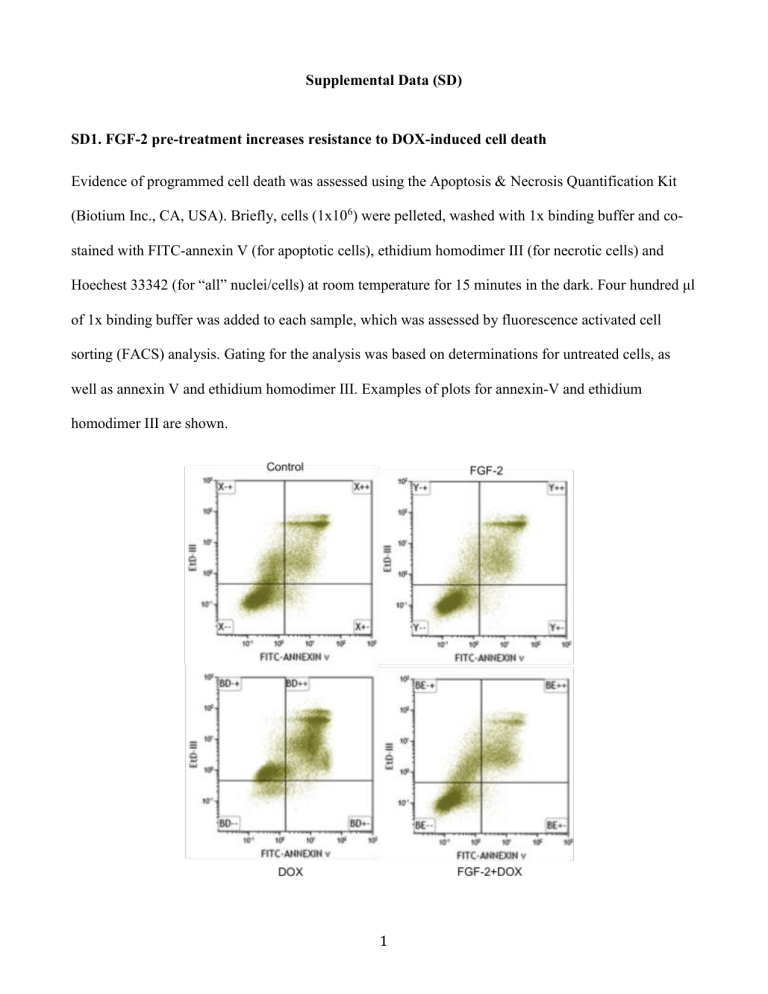
SD1. FGF-2 pre-treatment increases resistance to DOX-induced cell death
Evidence of programmed cell death was assessed using the Apoptosis & Necrosis Quantification Kit
(Biotium Inc., CA, USA). Briefly, cells (1x10
6
) were pelleted, washed with 1x binding buffer and costained with FITC-annexin V (for apoptotic cells), ethidium homodimer III (for necrotic cells) and
Hoechest 33342 (for “all” nuclei/cells) at room temperature for 15 minutes in the dark. Four hundred μl of 1x binding buffer was added to each sample, which was assessed by fluorescence activated cell sorting (FACS) analysis. Gating for the analysis was based on determinations for untreated cells, as well as annexin V and ethidium homodimer III. Examples of plots for annexin-V and ethidium homodimer III are shown.
1
SD2. (A) Melting curve for primers used for qPCR, and (B) assessment of MRP2 by PCR and agarose gel electrophoresis
A
2
B
As the melting curve for MRP2 suggested the possibility of a minor primer dimer, agarose gel electrophoresis was done and the presence of one major product of the expected size (318 bp) for
MRP2 was detected. MRP2 is expressed at relatively low levels in heart tissue compared to the other transporters tested, and was not detected in our control or FGF-2 treatment alone samples. The product was detected after DOX treatment (with and without FGF-2 treatment), but again at low levels.
3
SD3. FGF-2 pre-treatment increases resistance to DOX-induced relative cell loss
Cardiomyocytes were pelleted and nuclei stained with Hoechst 33342 and total cell number counted at
6 and 24 hours. The results are expressed as the mean plus or minus standard error of the mean, relative to the control (untreated) value, which is arbitrarily set to 100. Data were assessed by two-way
ANOVA with the Bonferonni post-test. A value of p<0.05 is considered statistically significant: p<0.01** /## , p<0.001, *** /### .
4
SD4. FGF-2 RNA level is decreased with 0.5 µM DOX treatment
The effect of DOX on FGF-2 RNA expression in cardiomyocyte cultures was assessed by qPCR after 6 and 24 hours. The results are expressed as the mean plus or minus standard error of the mean, relative to the control (untreated) value, which is arbitrarily set to 1. Data were assessed by t-test. A value of p<0.05 is considered statistically significant: p<0.05, *
5
SD5. FGF-2 has a positive effect on calcein efflux
Efflux transport function of fluorescent calcein was assessed using the Multi-Drug Resistance Assay
Kit (Calcein AM) according to the manufacturer’s instructions (Cayman Chemical Company, Ann
Arbor, USA). Briefly, cultures were treated with DOX and/or FGF-2, or transporter inhibitors (e.g., 20
µM cyclosporine A, 2 µM verapamil or 1 µM XR9576) as a positive control for efflux inhibition. To load cells with calcein, cultures were treated with cell-permeable non-fluorescent substrate calcein AM solution 25 minutes prior to harvesting. Following intracellular esterase activity that removes the acetomethoxy (AM) group, calcein AM is converted to fluorescent calcein, which is retained in the cytoplasm
1
. Cells were pelleted, resuspended in Assay Buffer and analyzed by FACS as described
(MoFloXDP, Beckman Coulter, Mississauga, Canada) within 1 hour of staining. Data were analyzed using the Summit v.5.2 program (Beckman). Gating for the analysis was based on determinations for untreated cells and calcein AM positive cells. Examples of histograms of calcein levels are shown.
6
SD6. FGF2 has a positive effect on intracellular DOX levels
(A) Effects on intracellular DOX concentration were also assessed by FACS using the autofluorescence properties of DOX
2
. Briefly, cardiomyocytes were treated with DOX and/or FGF-2, or 20 µM CsA or
2 µM verapamil as a positive control for efflux inhibition in medium (DMEM/F-12) without phenol red. Cells were pelleted and resuspended in the same medium for FACS analysis with
Ex and
Em of
488 and 585 nm as described
3
. Examples of histograms of DOX autofluorescence levels are shown.
7
(B) Standard curve of autofluorescence levels in relation to DOX concentration were analyzed, and the median intracellular concentration of DOX for each treatment was calculated using the software
Graphpad Prism.
References for SD
1. Orchard A, Schamerhorn GA, Calitree BD, Sawada GA, Loo TW, Claire Bartlett M et al.
Thiorhodamines containing amide and thioamide functionality as inhibitors of the ATP-binding cassette drug transporter P-glycoprotein (ABCB1). Bioorg Med Chem 2012;20:4290-4302.
2.
3.
Karukstis KK, Thompson EH, Whiles JA, Rosenfeld RJ. Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys Chem 1998;73:249-263.
Hasinoff BB, Patel D, Wu X. The Dual-Targeted HER1/HER2 Tyrosine Kinase Inhibitor
Lapatinib Strongly Potentiates the Cardiac Myocyte-Damaging Effects of Doxorubicin.
Cardiovasc Toxicol 2012.
8














