Introduction to Thermodynamics
advertisement
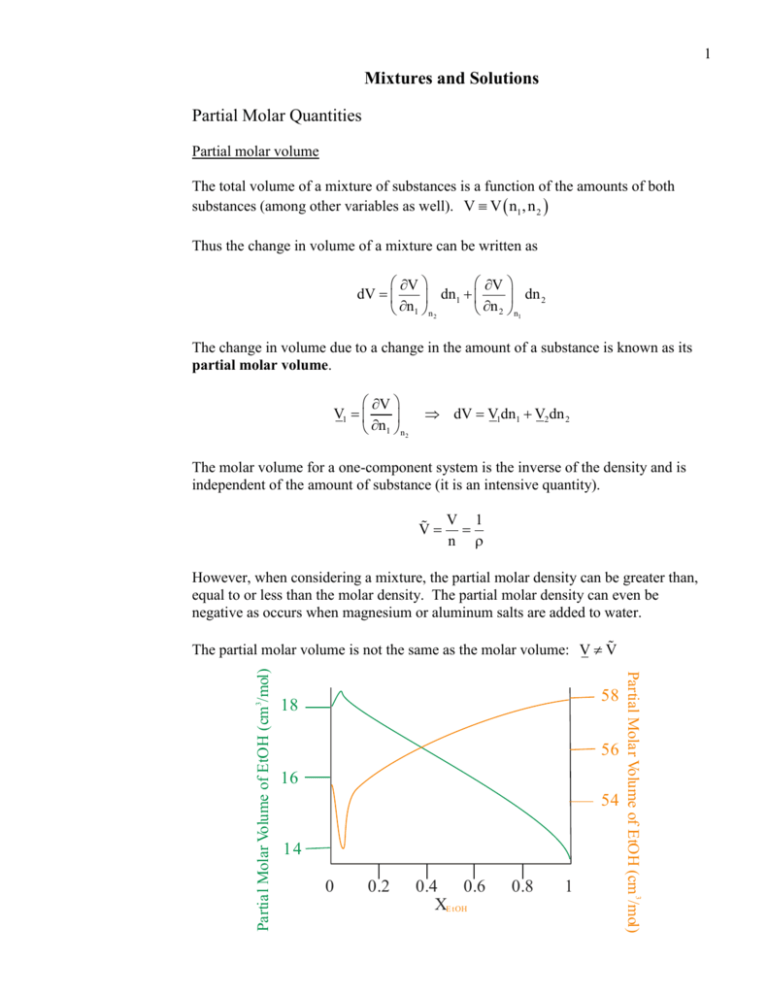
1 Mixtures and Solutions Partial Molar Quantities Partial molar volume The total volume of a mixture of substances is a function of the amounts of both substances (among other variables as well). V V n1 , n 2 Thus the change in volume of a mixture can be written as V V dV dn1 dn 2 n1 n 2 n 2 n1 The change in volume due to a change in the amount of a substance is known as its partial molar volume. V V1 n1 n 2 dV V1dn1 V2 dn 2 The molar volume for a one-component system is the inverse of the density and is independent of the amount of substance (it is an intensive quantity). V V 1 n However, when considering a mixture, the partial molar density can be greater than, equal to or less than the molar density. The partial molar density can even be negative as occurs when magnesium or aluminum salts are added to water. 58 18 56 16 54 14 0 0.2 0.4 0.6 XE tOH 0.8 1 Partial Molar Volume of EtOH (cm 3/mol) Partial Molar Volume of EtOH (cm 3/mol) The partial molar volume is not the same as the molar volume: V V 2 Example: Calculate the total volume of solution when 0.050 mol of MgSO4 is added to 1.00 kg of water. The partial molar volume of MgSO4 depends on its concentration with the following expression: VH2O 18.063 cm3/mol VMgSO4 69.38 m m 0.070 cm3 mol V VMgSO4 n MgSO4 VH2O n H2O 69.38 0.050 m m 0.070 cm3 mol 0.050 mol 18.063cm3 mol 1000 g 18.02 g mol 0.069 cm3 1002.38cm3 1002.31cm3 Other partial molar quantities Other quantities such as partial molar heat capacities are useful for thermodynamic work with solutions. Cp Cp1 n1 n 2 ,n3 ,n3 Partial molar Gibbs free energy As seen in the last chapter, the partial molar Gibbs free energy at constant temperature and pressure is the chemical potential G 1 n1 T,p,n 2 ,n3 , So the total Gibbs free energy of a mixture can be found in terms of the chemical potential. G 1n1 2 n 2 3n 3 4 n 4 3 Gibbs – Duhem equation The chemical potentials of the components of a mixture are not independent of each other. Change in the chemical potential depends on the changes of the other chemical potentials. Let us examine the Gibbs free energy of a two-component mixture. G 1n1 2 n 2 The change in the Gibbs free energy (at constant temperature and pressure) can be written in terms of changes in the chemical potentials and changes in the amounts. dG d 1n1 2 n 2 n1d1 1dn1 n 2d2 2dn 2 However recall that the Gibbs free energy is a function of temperature, pressure and amount. G G p, T, n1 , n 2 dG Vdp SdT 1dn1 2dn 2 dG T,p 1dn1 2dn 2 Comparing the two expressions for dG leads to the Gibbs – Duhem equation. n1d1 1dn1 n 2d 2 2dn 2 1dn1 2dn 2 n 2 d 2 n1 Similar relationships hold for the partial molar volumes and partial molar entropies of a mixture. n1d1 n 2 d 2 0 d1 dV1 n 2 dV2 n1 dS1 n 2 dS2 n1 Changes in one partial molar quantity depend on the changes of the other partial molar quantities. 4 Activities and ideal solutions Consider a solution of a volatile solvent with a nonvolatile solute. It is an experimental fact that the vapor of the solvent above the solution will be reduced as more solute is added to the solution. For the solvent at equilibrium, the chemical potential of the vapor is equal to the chemical potential of the liquid. (“A” is a customary label for the solvent when examining solutions.) A g A l The chemical potential of the vapor is generally lower than its standard state. p A g A g RT ln A p Thus the chemical potential of the solvent in solution can be written in terms of the chemical potential of its vapor. p A l A g RT ln A p For a pure solvent, a similar expression can be written for the solvent vapor in equilibrium with the pure liquid. (The “*” indicates pure solvent.) p* *A l A g RT ln A p Examining the difference between the chemical potential of the liquid in solution and the chemical potential of the pure liquid yields p*A g RT ln A p p p* p RT ln A RT ln A RT ln *A p p pA p A l *A l A g RT ln A p The difference in the chemical potential of the solvent can be expressed in terms of activity. p A l *A l RT ln *A RT ln a A pA 5 Thus activity is A l *A l p ln a A a A *A RT pA If the activity of the solvent in the solution is equal to the mole fraction, the solution is called an ideal solution as it follows Raoult’s law. aA xA PA pA x p * A A PA0 Properties of ideal solution Volume G Recall that V p Then 0 XA 1 1 G G 1 V V1 V1 n1 p p n1 p n1 p A l *A l RT ln x A A l *A l RT ln x A p A l *A l p V1 V1* RT ln x A RT ln x A p p ln x A 0 V1 V1* 0 V1 V1* p The equality of the partial molar volumes implies that in an ideal solution, all the molecules are the same size. 6 Enthalpy Recall the Gibbs-Helmholtz equation: G H G H 2 n1 T p T n1 T 2 T p T T H G 1 21 T T p T H 1 21 T T p T * H A H*A RT ln x A RT ln x A A l A l 2 T T T T T ln x A 0 H1 H1* 0 H1 H1* T The equality of the partial molar enthalpies implies that in an ideal solution, all the interactions between molecules are the same. That is, Hsolvent solvent Hsolutesolvent Hsolutesolute Aside: Very often the solute – solute interactions are assumed to be zero. Hsolutesolute 0 Compare the microscopic conditions of an ideal solution to that of an ideal gas. volume intermolecular forces ideal solution nonzero, all the same nonzero, all the same ideal gas none none 7 Thermodynamics of Mixing Gibbs free energy of mixing The chemical potential of a substance changes when it is part of a solution and the difference between the two chemical potentials is what is used to define the activity. Let G be the Gibbs free energy of a mixture G n11 n 22 n 33 G* be the Gibbs free energy of all of the components before mixing. G* n11* n 2*2 n 3*3 Then the Gibbs free energy of mixing can be defined as the difference between two quantities. G mix G G* n i i *i n i RT ln a i nRT x i ln a i i i i The Gibbs free energy can be rewritten in terms of the mole fraction by recalling that n i n x i , where n is the total number of moles of all substances in the solution. G mix RT n i ln a i nRT x i ln a i i If the solution is ideal, then i G mix nRT x i ln x i i Entropy of mixing G Recall that S T p Let us taking the derivative of the Gibbs free energy of mixing to find the entropy of mixing. G mix nRT x i ln x i T i G mix nRT x i ln x i T T i Smix nR x i ln x i i 8 Enthalpy of mixing Recall the Gibbs – Helmholtz equation G H 2 T p T T Apply the equation to the Gibbs free energy of mixing. G mix T nRT x i ln x i i T nR x i ln x i Smix i G mix H mix nR x i ln x i 0 2 T T T T i H mix 0 H mix 0 T2 Therefore an ideal solution has no enthalpy of mixing. The mixing of an ideal solution is strictly an entropic process. Colligative Properties Colligative properties are properties of solutions that depend on the amount of solute and not the identity of the solute (at first approximation). Adding solute decreases the chemical potential of the solvent. A l *A l RT ln a A RT ln x A Lower the chemical potential of a liquid component in a solution, lowers the freezing point and raises the boiling point. solid liquid solution gas Tm Tm TbTb T 9 Freezing point depression At equilibrium (at the melting point) 1 l 1 s [Subscript “1” labels the solvent.] The chemical potential of the liquid is related to its mole fraction in solution. A l *A l RT ln x A 1 s 1* l RT ln x1 1* l 1 s ln x1 RT However, consider that G fus 1* l 1 s G fus ln x1 RT Take the temperature derivative, considering the Gibbs – Helmholtz equation. G fus ln x1 T RT T ln x1 1 G fus R T T T ln x1 H fus T RT 2 ln x1 1 H fus 2 R T T Integrate the equation assuming Hfus is constant. (Excellent assumption considering the temperature range involved.) Tm* 1 Hfus Hfus 1 1 dT ln1 ln x1 2 * RT R Tm Tm Tm d ln x 1 x1 ln x1 H fus 1 1 * R Tm Tm Change the emphasize from the amount of solvent to the amount of solute (labeled “2”), remembering that x1 x 2 1 ln 1 x 2 Hfus Tm Tm* R Tm* Tm Assume that Tm* Tm so that Tm* Tm Tm*2 Also, from a Taylor series, ln 1 x 2 x 2 Hfus Tm Tm* x 2 R Tm*2 10 Rearranging yields Tm* Tm x 2 RTm*2 H fus Consider a relatively dilute solution such that n2 << n1. Then x 2 n 2 n1 The moles of solute can be calculated in terms of molality (c2) and mass of the solvent, n 2 c2 m1 . The moles of solvent can be calculated in terms of mass of the solvent and its molar mass, n1 m1 M1 . Thus Tm x 2 RTm*2 n 2 RTm*2 c2 m1RTm*2 c M RT*2 2 1 m H fus n1H fus m1 M1 H fus H fus M1RTm*2 Tm c2 k f c2 H fus The kf is called the cryoscopic constant and it is unique for each solvent. kf M1RTm*2 H fus At equilibrium (at the boiling point) 1 l 1 v From here, a similar analysis can be done to find the boiling point elevation as Tb k bc2 where kb, the ebullioscopic constant, is kb M1RTb*2 H vap 11 Osmotic pressure Osmosis – solutions in contact with a semipermeable membrane that allows flow of only solvent through the membrane. Osmosis At equilibrium, the chemical potential of the solution “on the left” of the semipermeable membrane is equal to the chemical potential of the solution “on the right” of the membrane. Assume the solution “on the right” is actually the pure solvent. 1 T, patm , x1 1* T, patm The chemical potential on the left has a concentration dependence. 1 T, patm , x1 1* T, patm RTln x1 Thus, 1* T, patm 1* T, patm RT ln x1 1* T, patm 1* T, patm RTln x1 0 The difference in chemical potentials can be cleverly rewritten as an integral. 1* T, patm 1* T, patm patm d1* T patm G However, since V V p T p T patm patm d T RT ln x1 0 * 1 patm d Vdp V1*dp RT ln x1 0 V1*p patm V1* patm patm RTln x1 V1* RTln x1 0 Recall our trick with the freezing point depression, ln x1 ln 1 x 2 x 2 x 2 patm patm RT ln x1 0 12 V1* RT ln x1 RTx 2 RT V1* RT n2 n1 n2 n1 V1*n1 n 2 RT V n 2 RT n2 RT V c RT Another one of the van’t Hoff equations. The special case of ionic solutions Because colligative properties are independent of the specific nature of the solute, no distinction is made between a molecule in solution and an ion in solution. Thus, when taking into account the colligative properties of ionic solutions, the dissociation of the ionic compound must be considered. 0.12 M NaCl 0.12 M Na 0.12 M Cl 0.24 M particles 0.45M K2SO4 0.90 M K 0.45M SO2 1.35M particles 4 The proper way to write the van’t Hoff equation for osmotic pressure is i c RT where “i” is the called the van’t Hoff factor which describes the amount of dissociation of electrolytes (strong and weak). Example: 4.02 mg of human insulin is dissolved in 10.7 mL of water. The osmotic pressure of the solution is 1.25 torr at 37 C. Calculate the molar mass of the protein. Calculate concentration of solute 1atm 760 torr c 6.47 x105 M 0.08206 L atm RT 310 K mol K 1.25 torr Calculate moles of solute n c V 6.47 x105 mol 0.0107 L 6.92 107 mol L Calculate molar mass 4.02x10 3 g m M 4160g mol n 6.92x10 7 mol







