(Z)- and - Georgia Gwinnett College
advertisement

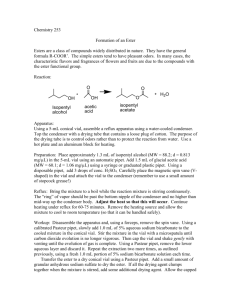
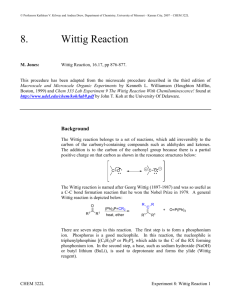
Organic Chemistry II Lab CHEM 2212L (Fall 2008) School of Science and Technology Georgia Gwinnett College Experiment I. Synthesis of (Z)- and (E)-Stilbenes by a Wittig Reaction 1. EXPERIMENTAL OBJECTIVES: a. Synthesize a mixture of stilbenes by a phase-transfer Wittig reaction. b. Isolate the product. c. Characterize and analyze. d. Present, analyze, and discuss results in a written report. 2. SPECIAL INSTRUCTIONS: a. Chemicals and solvents: (1) 50% NaOH, [1310-73-2] (2) NaHSO3, [7631-90-5] (3) benzyltriphenylphosphonium chloride, [1100-88-5] (4) benzaldehyde, [100-52-7] (5) CH2Cl2, [75-9-2] (6) CH3CH2OH, [64-17-5] (7) Na2SO4, [7757-82-6] (8) Z/E-stilbene, [103-30-0] b. Two products are possible in this reaction. Which is the major product? Why? See Scheme 18-1, pg. 588. c. As you prepare your separation flow chart (which is critical for your success), note that the "Work-Up, Isolation, and Purification" section of the procedures, the “washing” steps are similar to the “extractions” you did in Experiment H. It is critical that you determine which layer is on top, organic or aqueous, at each step of the process. 3. PROCEDURES: 1. During the first lab period, you will perform the synthesis of Z/E-stilbene, work-up and isolation to include drying the product/dichloromethane solution with anhydrous sodium sulfate. Note below that we are skipping the isomerization step. You will leave your product in a 3 mL conical vial uncapped until the next lab period. 2. The second lab period is for crude recrystallization and analysis via % yield, IR, and mp. Page 1 of 2 2/12/16 Organic Chemistry II Lab CHEM 2212L (Fall 2008) School of Science and Technology Georgia Gwinnett College Setting Up 3. Do not prepare a 50% NaOH solution; use the solution provided. You do not need the drying tube as shown in the sketch on the top of p. 592, just set up the condenser for refluxing. 4. Weigh the benzyltriphenylphosphonium chloride. Use a plastic pipet to obtain ~ 2 mL dichloromethane. Measure the benzaldehyde with the ADP. Use the smallest stir bar. Wittig Reaction 5. Once gently refluxing (sandbath, heat setting of 2-3), add the NaOH dropwise using a glass pipet (~40 drops or one “squeeze on the bulb” ~ 1 mL) through the condenser to avoid splashing. The reaction is vigorous! Keep the reflux gentle and ensure stirring for 30 minutes. 6. Do IR of starting material and set up for the washing/extracting/drying while the reaction is refluxing. Work-Up and Isolation of Isomeric Stilbenes 7. When the reaction time is done, keep the condenser operating and cool the connected reaction vial with an ice bath for a few minutes. 8. Follow the procedures through the point of “ … until the pH of the wash is neutral.” At this point, run your “wet” product/dichloromethane solution through a Pasteur filter tip pipet full of anhydrous sodium sulfate and into your 3 mL conical vial. 9. Store your “dry” product/dichloromethane in the open 3 mL conical vial until the next lab period. 10. You will not do 1H NMR of the crude product mixture. Isomerization 11. You will not do the isomerization. Work-Up and Isolation of Stilbene 12. You will begin the second lab period at the last paragraph on p. 592. You will not do 1H NMR. Start with “Dissolve the remaining thick, …” Use filter paper to capture your crude recrystallized product. Dry this product under the heat lamp for about 15-20 minutes, which will produce the dry crystalline flakes. You will not do the last sentence of the procedures, which is to do a Craig Tube recrystallization of the isolated product. Analysis 13. Determine product % yield, IR, and melting point. Conduct standard analysis of experimental data, comparing your results to literature values. 14. Perform the Bromine test for unsaturation (Sec. 25.8, p. 851). Page 2 of 2 2/12/16