How Control Agents Affect Bacteria

II. THE PROKARYOTIC CELL:
BACTERIA
D. CONTROL OF BACTERIA
1. How Control Agents Affect Bacterial
Structures and Functions
The overall purpose of this Learning Object is:
1) to learn how our antibacterial control agents affect bacteria by altering their cellular structures or interfering with their cellular functions; and
2) to introduce a variety of chemical agents frequently used to control bacterial growth.
LEARNING OBJECTIVES FOR THIS SECTION
The basis of chemotherapeutic control of bacteria is selective toxicity (def) .
Selective toxicity means that the chemical being used should inhibit or kill the intended pathogen without seriously harming the host. A broad spectrum agent
(def) is one generally effective against a variety of gram-positive and gramnegative bacteria; a narrow spectrum agent (def) generally works against just gram-positives, gram-negatives, or only a few bacteria. Such agents may be cidal or static in their action. A cidal (def) agent kills the organism while a static
(def) agent inhibits the organism's growth long enough for body defenses to remove it. There are two categories of antimicrobial chemotherapeutic agents: antibiotics and synthetic drugs. Antibiotics (def) are metabolic products of one microorganism that inhibit or kill other microorganisms. Synthetic drugs (def) are antimicrobial drugs synthesized by chemical procedures in the laboratory.
Many of today's antibiotics are now actually semisynthetic and some are even made synthetically.
We will now look at the two sides of the story with regards to controlling bacteria by means of chemicals:
1. How Control Agents May Affect Bacterial Structures or Functions
2. How Bacteria May Resist Our Control Agents
We will now look at the various ways in which our control agents affect bacteria altering their structures or interfering with their cellular functions.
How Control Agents May Affect Bacterial Structures or
Functions
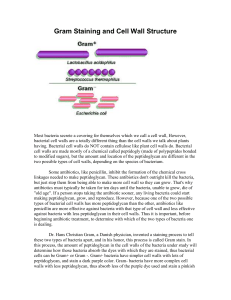
(see Fig. 1) a. Many antibiotics inhibit normal synthesis of peptidoglycan (def) by bacteria and cause osmotic lysis.
For More Information: Peptidoglycan from Unit 1.
In order for bacteria to increase their size following binary fission, links in the peptidoglycan must be broken, new peptidoglycan monomers must be inserted, and the peptide cross links must be resealed.
New peptidoglycan synthesis occurs at the cell division plane by way of a collection of cell division machinery known as the divisome .
The following sequence of events occur at the divisome:
1. Bacterial enzymes called autolysins: a) break the glycosidic bonds between the peptidoglycan monomers at the point of growth along the existing peptidoglycan (see Fig. 5, steps 1-3) ; and b) break the peptide cross-bridges that link the rows of sugars together (see Fig. 5, steps
1-3) .
2. Transglycosidase enzymes then insert and link new peptidoglycan monomers into the breaks in the
peptidoglycan (see Fig. 6, step 1 , Fig.
6, step 2) , and Fig. 6, step 3) .
3. Finally , transpeptidase enzymes reform the peptide cross-links between the rows and layers of peptidoglycan to make the wall strong
(see Fig. 6, step 4) .
Animation showing the synthesis of peptidoglycan.
Interference with this process results in a weak cell wall and osmotic lysis of the bacterium. Examples include the penicillins (penicillin G, methicillin, oxacillin, ampicillin, amoxicillin, ticarcillin, etc.), the cephalosporins (cephalothin, cefazolin, cefoxitin, cefotaxime, cefaclor, cefoperazone, cefixime, ceftriaxone, cefuroxime, etc.), the carbapenems
(imipenem, metropenem), the monobactems
(aztreonem), the carbacephems (loracarbef), and the glycopeptides (vancomycin, teichoplanin).
1. For example, penicillins and cephalosporins bind to the transpeptidase enzymes (also called penicillin-binding proteins) responsible for resealing the cell wall as new peptidoglycan monomers are added during bacterial cell growth. This blocks the transpeptidase enzymes from cross-linking the sugar chains and results in a weak cell wall and subsequent osmotic lysis of the bacterium
(see Fig. 6) .
Animation illustrating how penicillins inhibit peptidoglycan synthesis.
2. Glycopeptides like vancomycin bind to the peptides of the peptidoglycan monomers and block the formation of gycosidic bonds between the sugars by the transgycosidase enzymes, as well as the formation of the peptide cross-links by the transpeptidase enzymes.This results in a weak cell wall and
subsequent osmotic lysis of the bacterium (see
Fig. 7) .
Animation illustrating how vancomycins inhibit peptidoglycan synthesis.
3. Bacitracin prevents the peptidoglycan monomers synthesized in the cytosol from being transported across the cytoplasmic membrane .
As a result, no building blocks are available for peptidoglycan synthesis
(see Fig. 8) .
To view a Quick Time video of penicillin killing a bacterium , see the CELL'S ALIVE web page. To view articles on penicillin and antibiotics , see J.Brown's
Bugs in the News web page ay the University of
Kansas. b. INH (isoniazid) block the incorporation of mycolic acid into acid-fast cell walls (see Fig. 3) while ethambutol interferes with the synthesis of other cell wall components thus inhibiting cell wall synthesis. c. A very few antibiotics, such as polymyxins and tyrocidins as well as many disinfectants (def) and antiseptics (def) , such as orthophenylphenol, chlorhexidine, hexachlorophene, zephiran, alcohol, triclosans, etc., used during disinfection (def) alter the microbial cytoplasmic membranes (def) and cause leakage of cellular needs (see Fig. 1) .
For More Information: Cytoplasmic
Membrane from Unit 1.
d. Some antimicrobials inhibit normal nucleic acid replication in bacteria (see Fig. 1) .
1. The fluoroquinolones (norfloxacin, lomefloxacin, fleroxacin, ciprofloxacin, enoxacin, trovafloxacin, gatifloxacin, etc.) work by inhibiting one or more of a group of enzymes called
topoisomerase (def) , enzymes needed for bacterial nucleic acid synthesis. For example, DNA gyrase (topoisomerase
II), mentioned earlier in this unit, breaks and rejoins the strands of bacterial DNA to relieve the stress of the unwinding of
DNA that occurs during DNA replication and transcription (def) .
For More Information: The Nucleoid from Unit 1.
2. The sulfonamides and trimethoprim (co-trimoxazole - a combination of sulfamethoxazole and trimethoprim, sulfanilamide, etc.) block enzymes in the bacteria pathway required for the synthesis of tetrahydrofolic acid, a cofactor needed for bacteria to make the nucleotide bases thymine, guanine, uracil, and adenine (see Fig. 4) . This is done through a process called competitive antagonism whereby a drug chemically resembles a substrate in a metabolic pathway. Because of their similarity, either the drug or the substrate can bind to the substrate's enzyme. While the enzyme is bound to the drug, it is unable to bind to its natural substrate and that blocks that step in the metabolic pathway.
Sulfonamides such as sulfamethoxazole tie up the first enzyme in the pathway, the conversion of para-aminobenzoic acid to dihydropteroic acid.
Trimethoprim binds to the third enzyme in the pathway, an enzyme that is responsible for converting dihydrofolic acid to tetrahydrofolic acid. Without the tetrahydrofolic acid, the bacteria can not synthesize DNA or RNA.
3. Metronidazole is a drug that is activated by the microbial proteins flavodoxin and feredoxin found in
microaerophilc and anaerobic bacteria and certain protozoans. Once activated, the metronidazole puts nicks in the microbial DNA strands .
e. Rifampin blocks transcription (def) by inhibiting bacterial RNA polymerase, the enzyme responsible for transcription of DNA to mRNA.
For More Information: Transcription from Unit 4 f. Many antibiotics alter bacterial ribosomes (def) , interfering with translation (def) and thereby causing faulty protein synthesis (see Fig. 1) . To learn more detail about the specific steps involved in translation during bacterial protein synthesis, see the animation that follows. Protein synthesis will be discussed in greater detail in Unit 4.
For More Information: Ribosomes from
Unit 1
For More Information: Translation from
Unit 4
Animation illustrating the early stages of translation during bacterial protein synthesis.
1. The aminoglycosides (streptomycin, neomycin, netilmicin, tobramycin, gentamicin, amikacin, etc.) bind irreversibly to the 30S subunit of bacterial ribosomes and prevent the
50S ribosomal subunit from attaching to the translation initiation complex.
By binding to the 30S subunit, the subunit to which mRNA attaches, aminoglycosides also cause the misreading of the codons (see Fig. 5) resulting in tRNA inserting the wrong amino acids into the protein.
Animation illustrating how aminoglycosides bind to the 30S
ribosomal subunit and block translation.
2. The tetracyclines (tetracycline, doxycycline, demeclocycline, minocycline, etc.) bind reversibly to the 30S subunit , distorting it in such a way that the anticodons of charged tRNAs (def) cannot align properly with the codons of the mRNA (def) .
Animation illustrating how tetracyclines bind to the 30S ribosomal subunit and block translation.
3. The macrolides (erythromycin, azithromycin, clarithromycin, dirithromycin, troleandomycin, etc.) bind reversibly to the
50S subunit .They inhibit elongation of the protein by the enzyme peptidyltransferase that forms peptide bonds between the amino acids, by preventing the ribosome from translocating down the mRNA (def) , or both .
Animation illustrating how macrolides bind to the 50S ribosomal subunit and block translation by blocking peptidyltransferase.
Animation illustrating how macrolides bind to the 50S ribosomal subunit and block translation by blocking ribosome translocation.
4. The oxazolidinones (linezolid) bind to the
50S ribosomal subunit appear to interfere with the initiation of translation (def) .
5. The streptogramins (a combination of quinupristin and dalfopristin) bind to different sites on the 50S ribosomal subunit and work synergistically to inhibit translocation . g. Many disinfectants (chlorine, iodine, iodophores, mercurials, silver nitrate, formaldehyde, ethylene oxide, etc.) inactivate bacterial enzymes and thus block metabolism .
For a more detailed description of any specific antimicrobial agent, see the website of RxList - The Internet Drug Index .
For further information on the modes of action of antibiotics, see:
- the online textbook Microbiology Webbed Out at the University of Wisconsin-Madison
- the online microbook at the University of
Texas Medical Branch
QUIZ YOURSELF ON THIS SECTION
RETURN TO UNIT 1 TABLE OF CONTENTS
Doc Kaiser's Microbiology Home Page
Copyright © Gary E. Kaiser
All Rights Reserved
Updated: Oct. 6, 2004
Please send comments and inquiries to Dr. Gary Kaiser